Tricholeukaemia or hairy cell leukaemia
The information provided on www.fcarreras.org is intended to support, not replace, the relationship that exists between patients/visitors to this website and their physician.
Information endorsed by… ![]()
Information provided by Dr. Enric Carreras Pons. Doctor in Medicine and Surgery, Specialist in Internal Medicine, Specialist in Haematology and Haemotherapy and Medical Director of the Josep Carreras Foundation. Medical Association of Barcelona (Co. 9438).
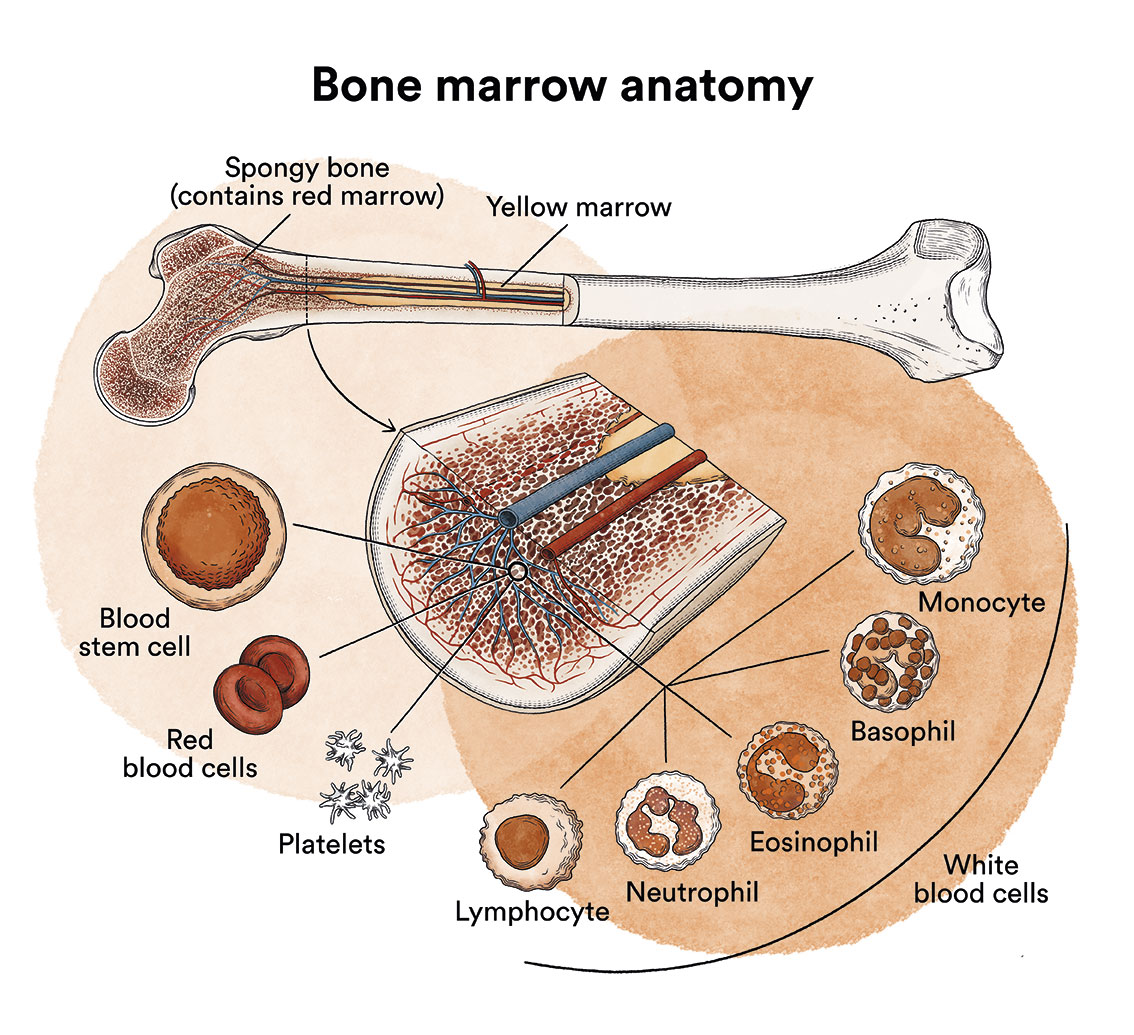
What is leukaemia, bone marrow and what are the types of blood cells?
Leukaemia is a type of cancer of the blood and bone marrow cells. See Leukaemia, bone marrow and blood cells.
What is tricholeukaemia or hairy cell leukaemia and who does it affect?
Tricholeukaemia (TL) also known as hairy cell leukaemia, is a rare and indolent leukaemia (slowly progressive), which starts in a particular subtype of B-lymphocyte (post-germinal centre memory lymphocytes). The name hairy cell leukaemia comes from the projections on the surface of the cells that look like hairs when examined under a microscope.
B-lymphocytes are white blood cells that help the body fight infection and are an essential part of the body’s immune system.
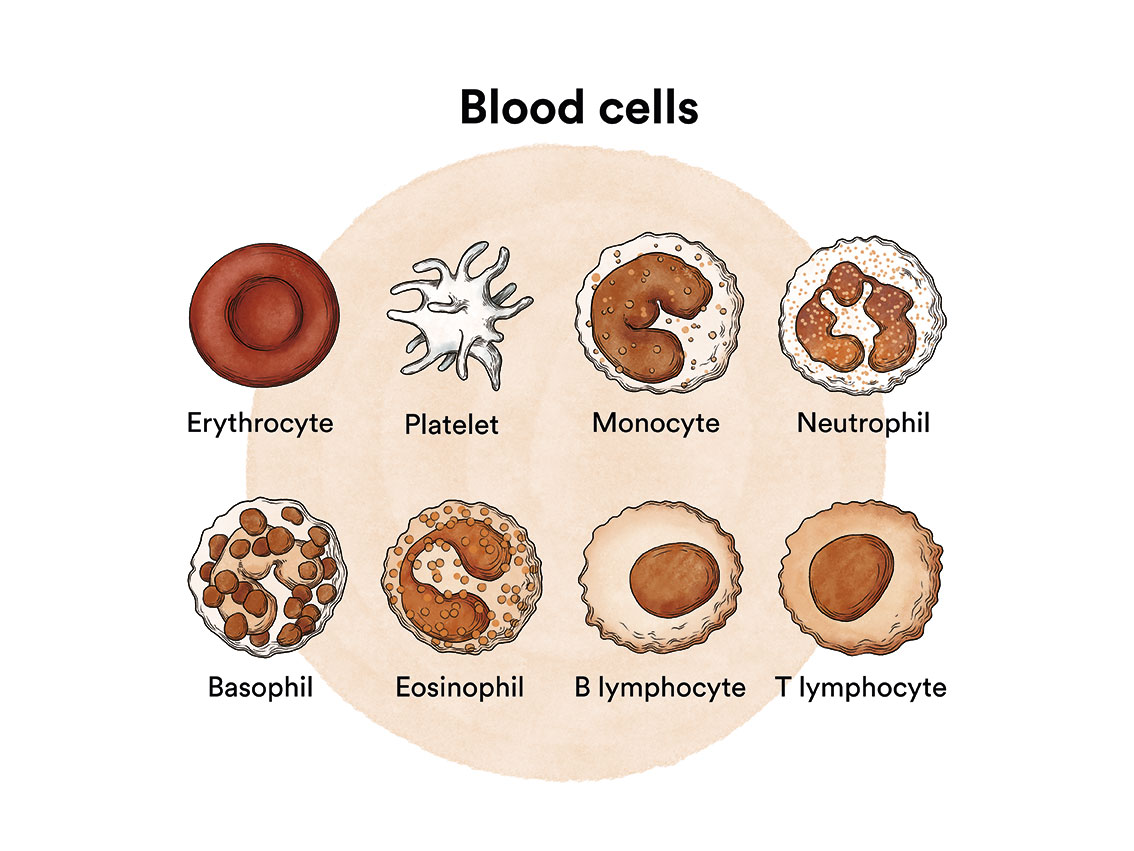
A mutation in one or more genes in a B-lymphocyte can cause it to become a leukaemic cell. A healthy B-lymphocyte, after a certain time, stops dividing and eventually dies. In tricholeukaemia, the mutations cause the B cell to continue to grow and divide. Since all the cells that arise from the initial leukaemic cell have the same alterations, they multiply uncontrollably. This proliferation causes them to infiltrate the bone marrow, spleen, and even the liver and lymph nodes.
Infiltration of the bone marrow (the soft, spongy tissue at the centre of most bones where blood cells are formed) affects the production of healthy blood cells. As leukaemic cells accumulate in the bone marrow, they inhibit the development of other blood cells, including red blood cells, platelets and white blood cells. As a result, there are too few functional normal cells due to an excess of leukaemic cells in the bone marrow. This can lead to a deficiency of blood cells which in turn can cause anaemia, excessive bleeding and/or infections.

Hairy cell leukaemia is rare. Between 2 and 4 cases per million population are diagnosed each year.
Hairy cell leukaemia variant
There are two types of hairy cell leukaemia, the typical hairy cell leukaemia described above and the so-called “variant”. Initially the variant was thought to be a subtype of hairy cell leukaemia. However, in 2008, the World Health Organization concluded that it is a distinct disease from hairy cell leukaemia and that there is no biological link between the two. It is rarer, has a different clinical course and is treated differently. It is usually seen in older patients (median of 71 years) and is not predominant in either sex.
What are the causes of hairy cell leukaemia or tricholeukaemia?
The specific causes of most cases of leukaemia in adults are not known. There are also some risk factors that are associated with a higher chance of developing leukaemia. A risk factor is anything that increases the likelihood that a person will develop cancer.
Risk factors associated with tricholeukaemia are:
- Age: much more common in adults aged 50-60.
- Gender: more common in men than women (4:1 ratio).
- Exposure to certain herbicides.
Leukaemia, like other cancers, is not contagious. See section Leukaemia, bone marrow and blood cells.
What are the symptoms of tricholeukaemia or hairy cell leukaemia?
The signs and symptoms of tricholeukaemia are totally non-specific and in up to 25% there are no symptoms at all and it is a casual laboratory finding during a routine blood test. The most common symptoms are attributable to insufficient bone marrow production: fatigue due to lack of red blood cells, bleeding due to lack of platelets, and infections due to lack of leukocytes. See section Leukaemia, bone marrow and blood cells. Occasionally fever, weight loss and pain under the ribs on the left side (due to enlargement of the spleen) may be observed.
Physical examination shows enlargement of the spleen in 90% of cases, enlargement of the liver (35%) or lymph nodes (up to 20% in the tricholeukaemia variant)
How is tricholeukaemia or hairy cell leukaemia diagnosed?
Diagnosis of this disease requires some experience as it is rare and can be mistaken for other blood diseases. As the symptoms and physical examination are non-specific, the diagnosis will be based on information provided by a number of laboratory tests:
– The haemogram will show altered (decreased) blood counts.
– Blood smear (microscopic examination of circulating blood) may show small to medium-sized leukaemic cells with hair-like projections.
– The bone marrow aspiration-puncture is performed through a hollow needle puncturing the hip bone (or sternum) to aspirate a liquid sample of cells.
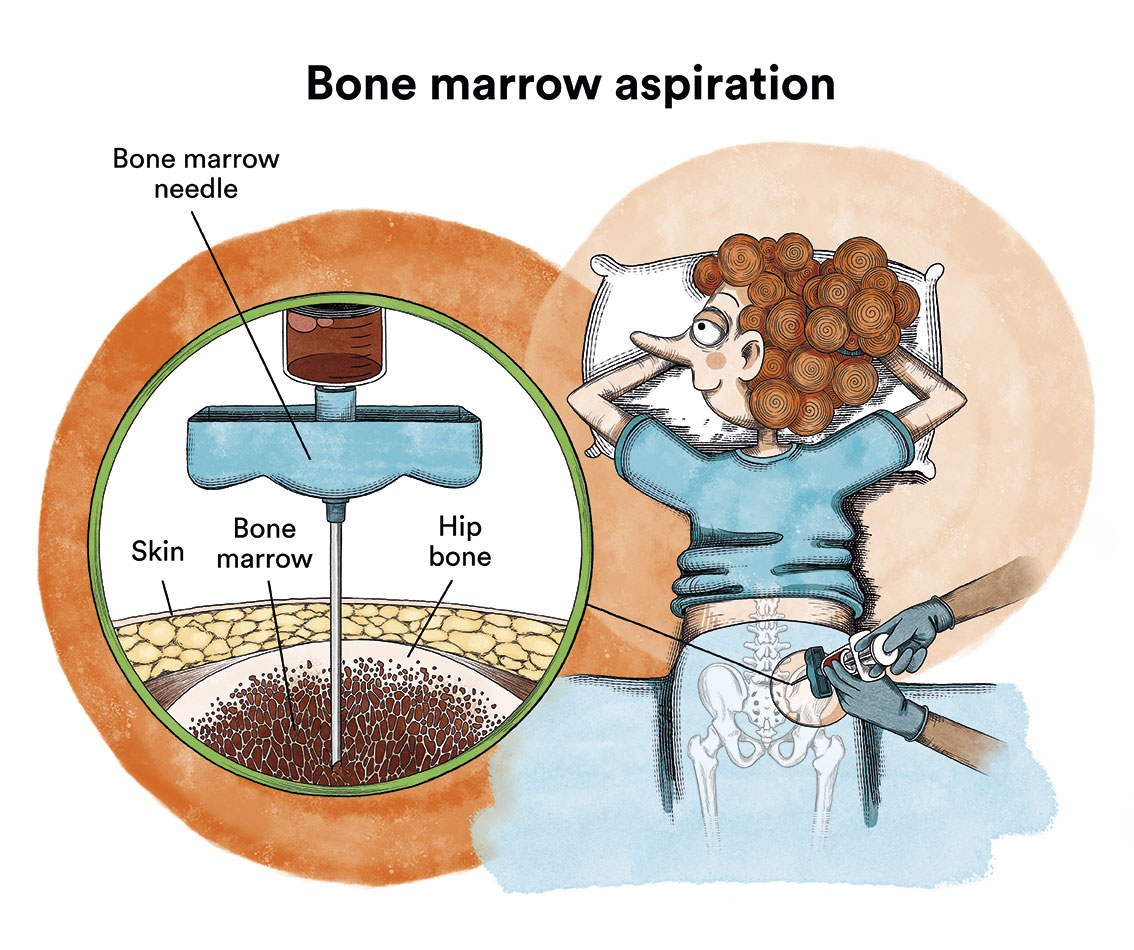
In these patients it is not uncommon for the bone marrow aspiration to be “dry” (the expected liquid sample is not obtained because the hairy cells often produce fibrous tissue that dries out the bone marrow). This problem is solved by bone marrow biopsy, which uses a special wider needle to extract a bone sample with its bone marrow.
For both products, a flow cytometry, a test that allows the cells present in the sample to be filtered, should always be performed. Since the proteins on the surface of hairy cells have a different characteristic arrangement than those of healthy B cells and other abnormal (malignant) B cells, their examination allows a diagnosis of certainty. Nowadays, all these studies must be complemented by molecular studies that study the presence of the characteristic genetic mutations of this disease.
In almost all cases of tricholeukaemia, the leukaemic cells have a mutation in the BRAF V600E gene. This mutation can serve as a molecular marker to differentiate this leukaemia from other leukaemias and lymphomas. Other mutations (such as the IGHV gene) may serve as factors in predicting the likely clinical outcome of the disease (prognosis). Thus, 90% of patients with this mutation will respond to classic treatments, so its presence implies a better prognosis.
Imaging tests such as computed tomography (or CT) are not diagnostic, but allow us to know the extent of the disease (affected organs) at diagnosis and thus monitor the response to treatment.
What is the treatment for tricholeukaemia or hairy cell leukaemia?
Based on symptomatology, blood work, imaging tests and presence or absence of major prognostic factors, treatment planning should be carried out.
There are essentially two options: the standard treatment or a clinical trial. Since hairy cell leukaemia is usually slowly progressive, not all patients need to start treatment after initial diagnosis. About 10-20% of patients have mildly altered blood tests and are asymptomatic and therefore it is recommended to “wait and see”, i.e. postpone the start of treatment until the appearance of symptoms or noticeable blood test abnormalities. It is relatively common for patients with tricholeukaemia to live for many years without any symptoms and without receiving any treatment. This behaviour requires a relatively frequent analytical follow-up adapted to the patient’s evolution.
In patients with notable analytical alterations or symptoms, initial treatment is usually with chemotherapeutic agents called purine analogues: cladribine (Leustatin®) and pentostatin (Nipent®). Both appear to be equally effective in achieving a durable remission. In 80-85% of patients, this involves normalisation of blood counts, reduction in spleen size and disappearance of symptoms, a response that can last for years. Since infections are the most common cause of problems in these patients, it should be noted that after receiving purine analogues, which are immunosuppressive agents, the risk of infections increases, so patients should be educated on how to prevent infections and warn the health care staff monitoring them about the presence of fever.

Treatment for relapsed or resistant cases
Treatment with purine analogues has improved the survival of patients with tricholeukaemia and some patients achieve remissions that last for years without the need for further treatment. However, some do not respond or have a very short response time. In these cases, additional treatments are needed:
In cases without response to purine analogues (initial resistance) the following options are available:
- The moxetumomab pasudotox-tdfk (Lumoxiti®).
- A different purine analogue, with or without rituximab (Rituxan®).
- Rituximab (if the patient cannot receive purine analogue therapy)
- Interferon alfa (Intron® A)
If there is a relapse (recurrence of the disease) the options are:
- Repeat the same initial treatment when the relapse occurs after more than 5 years of remission.
- The moxetumomab pasudotox-tdfk (LumoxitiTM), if the patient has received at least two prior systemic therapies, including treatment with a purine nucleoside analogue.
Patients with remissions that have lasted between two and five years may benefit from additional treatment with the same purine analogue, combined with rituximab, or from a treatment option available in a clinical trial.
If remission lasted less than two years, their options include:
- Treatment with an alternative purine analogue, associated with rituximab.
- Rituximab (if the patient cannot receive purine analogue therapy).
- Diagnostic tests to reconfirm the diagnosis of tricholeukaemia.
If the diagnosis is confirmed, the treatment option should be based on investigational agents available in clinical trials.
Investigational treatments
Each new drug or therapeutic regimen that is currently available undergoes a series of studies, called “clinical trials” before becoming part of standard treatment. Clinical trials are carefully designed and evaluated by expert clinicians and researchers to ensure the highest possible level of safety and scientific accuracy.
Participation in a carefully conducted clinical trial may be the best available treatment option. Patient participation in previous clinical trials has led to the development of the therapies we have today. The most notable are:
- BRAF inhibitors. Protein produced by the BRAF mutation
- Vemurafenib (Zelboraf®)
- Dabrafenib (Tafinlar®)
- Cell receptor inhibitors
- Ibrutinib (Imbruvica®)
- Monoclonal antibody therapy. Agents designed to target specific types of cancer cells
- Rituximab (Rituxan®), alone or in combination with other drugs
- Immunotoxins, anticancer drugs consisting of monoclonal antibodies linked to toxins.
- Immunotoxin LMB-2 is in trial phase.
Follow-up
After completing the treatment, patients will have regular check-ups by their haematologist and other specialists on a case-by-case basis. The check-ups are carried out to evaluate a possible relapse and to monitor and treat possible long-term complications.
What are the chances of tricholeukaemia patients being cured?
Tricholeukaemia is classified as a “chronic” (or indolent) leukaemia, as its progression is usually slow and non-aggressive, but unfortunately there is currently no cure. In many patients, treatment with chemotherapy can lead to a remission that can last for years. However, despite major advances in disease control, many patients relapse after treatment and require additional therapy.
Links of interest concerning medical issues relating to tricholeukaemia
Hairy Cell Leukemia Treatment. National Cancer Institute
Understanding Hairy Cell Leukemia. Hairy Cell Leukemia Foundation
Hairy cell leukemia (HCL). Leukemia & Lymphoma Society
Links of interest on other topics related to leukaemia
TESTIMONIAL MATERIALS
You can order the booklets in paper format for free delivery in Spain by e-mail: imparables@fcarreras.es
BONE MARROW TRANSPLANT
- Bone Marrow Transplant Guide. Josep Carreras Foundation (content in Spanish)
- What is HLA and how does it work? Josep Carreras Foundation (content in Spanish)
- Graft-versus-Host Disease. Josep Carreras Foundation (content in Spanish)
- History of Bone Marrow Transplantation. Josep Carreras Foundation (content in Spanish)
- How is the search for an anonymous donor conducted? Josep Carreras Foundation (content in Spanish)
FOOD
- How to maintain a healthy diet during treatment? Josep Carreras Foundation (content in Spanish)
- Nutrition guide. Leukemia & Lymphoma Society
OTHER
- Ideas on what to take with me to the isolation chamber. Josep Carreras Leukaemia Foundation (content in Spanish)
- Travel tips for people with cancer. Josep Carreras Leukaemia Foundation (content in Spanish)
- Physiotherapy manual for haematological and transplant patients. Josep Carreras Leukaemia Foundation (content in Spanish)
- Prevention and treatment of oral mucositis. Josep Carreras Leukaemia Foundation (content in Spanish)
- Oral hygiene in oncohaematological patients. Josep Carreras Leukaemia Foundation (content in Spanish)
- Fertility manual: Suffering from blood cancer and becoming a parent. Josep Carreras Leukaemia Foundation (content in Spanish)
- Skin care in the oncohaematological patient. Josep Carreras Leukaemia Foundation (content in Spanish)
- Aesthetic Oncology Manual. Josep Carreras Leukaemia Foundation (content in Spanish)
- Leukaemia and sexuality. Josep Carreras Leukaemia Foundation (content in Spanish)
- 7 ways to wear a scarf. Josep Carreras Leukaemia Foundation (content in Spanish)
Links of interest: local/provincial or state entities that can provide you with resources and services specialised in leukaemia or cancer patients:
In Spain there is a large network of associations for haematological cancer patients that, in many cases, can inform you, advise you and even carry out certain procedures. These are the contacts for some of them by Autonomous Community:
All these organisations are external to the Josep Carreras Foundation.
STATE
- CEMMP (Comunidad Española de Pacientes de Mieloma Múltiple)
- AEAL (ASOCIACIÓN ESPAÑOLA DE AFECTADOS POR LINFOMA, MIELOMA y LEUCEMIA)
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch or call 900 100 036 (24h).
- AELCLES (Agrupación Española contra la Leucemia y Enfermedades de la Sangre)
- JOSEP CARRERAS LEUKAEMIA FOUNDATION
- FUNDACIÓN SANDRA IBARRA
- GEPAC (GRUPO ESPAÑOL DE PACIENTES CON CÁNCER)
- MPN España (Asociación de Afectados Por Neoplasias Mieloproliferativas Crónicas)
ANDALUCÍA
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- ALUSVI (ASOCIACIÓN LUCHA Y SONRÍE POR LA VIDA). Sevilla
- APOLEU (ASOCIACIÓN DE APOYO A PACIENTES Y FAMILIARES DE LEUCEMIA). Cádiz
ARAGÓN
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- ASPHER (ASOCIACIÓN DE PACIENTES DE ENFERMEDADES HEMATOLÓGICAS RARAS DE ARAGÓN)
- DONA MÉDULA ARAGÓN
ASTURIAS
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- ASTHEHA (ASOCIACIÓN DE TRASPLANTADOS HEMATOPOYÉTICOS Y ENFERMOS HEMATOLÓGICOS DE ASTURIAS)
CANTABRIA
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
CASTILLA LA MANCHA
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
CASTILLA LEÓN
- ABACES (ASOCIACIÓN BERCIANA DE AYUDA CONTRA LAS ENFERMEDADES DE LA SANGRE)
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- ALCLES (ASOCIACIÓN LEONESA CON LAS ENFERMEDADES DE LA SANGRE). León.
- ASCOL (ASOCIACIÓN CONTRA LA LEUCEMIA Y ENFERMEDADES DE LA SANGRE). Salamanca.
CATALUÑA
- ASSOCIACIÓ FÈNIX. Solsona
- FECEC (FEDERACIÓ CATALANA D’ENTITATS CONTRA EL CÁNCER
- FUNDACIÓ KÁLIDA. Barcelona
- FUNDACIÓ ROSES CONTRA EL CÀNCER. Roses
- LLIGA CONTRA EL CÀNCER COMARQUES DE TARRAGONA I TERRES DE L’EBRE. Tarragona
- MielomaCAT
- ONCOLLIGA BARCELONA. Barcelona
- ONCOLLIGA GIRONA. Girona
- ONCOLLIGA COMARQUES DE LLEIDA. Lleida
- ONCOVALLÈS. Vallès Oriental
- OSONA CONTRA EL CÀNCER. Osona
- SUPORT I COMPANYIA. Barcelona
- VILASSAR DE DALT CONTRA EL CÀNCER. Vilassar de Dalt
VALENCIAN COMMUNITY
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- ASLEUVAL (ASOCIACIÓN DE PACIENTES DE LEUCEMIA, LINFOMA, MIELOMA Y OTRAS ENFERMEDADES DE LA SANGRE DE VALENCIA)
EXTREMADURA
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- AFAL (AYUDA A FAMILIAS AFECTADAS DE LEUCEMIAS, LINFOMAS; MIELOMAS Y APLASIAS)
- AOEX (ASOCIACIÓN ONCOLÓGICA EXTREMEÑA)
GALICIA
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- ASOTRAME (ASOCIACIÓN GALLEGA DE AFECTADOS POR TRASPLANTES MEDULARES)
BALEARIC ISLANDS
- ADAA (ASSOCIACIÓ D’AJUDA A L’ACOMPANYAMENT DEL MALALT DE LES ILLES BALEARS)
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
CANARY ISLANDS
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- AFOL (ASOCIACIÓN DE FAMILIAS ONCOHEMATOLÓGICAS DE LANZAROTE)
- FUNDACIÓN ALEJANDRO DA SILVA
LA RIOJA
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
MADRID
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- AEAL (ASOCIACIÓN ESPAÑOLA DE LEUCEMIA Y LINFOMA)
- CRIS CONTRA EL CÁNCER
- FUNDACIÓN LEUCEMIA Y LINFOMA
MURCIA
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
NAVARRA
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
BASQUE COUNTRY
- AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present in the different provinces and in many municipalities. Contact the nearest branch.
- PAUSOZ-PAUSO. Bilbao
AUTONOMOUS CITIES OF CEUTA AND MELILLA
- AECC CEUTA (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER)
- AECC MELILLA (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER)
Support and assistance
We also invite you to follow us through our main social media (Facebook, Twitter and Instagram) where we often share testimonies of overcoming this disease.
If you live in Spain, you can also contact us by sending an e-mail to imparables@fcarreras.es so that we can help you get in touch with other people who have overcome this disease.
* In accordance with Law 34/2002 on Information Society Services and Electronic Commerce (LSSICE), the Josep Carreras Leukemia Foundation informs that all medical information available on www.fcarreras.org has been reviewed and accredited by Dr. Enric Carreras Pons, Member No. 9438, Barcelona, Doctor in Medicine and Surgery, Specialist in Internal Medicine, Specialist in Hematology and Hemotherapy and Senior Consultant of the Foundation; and by Dr. Rocío Parody Porras, Member No. 35205, Barcelona, Doctor in Medicine and Surgery, Specialist in Hematology and Hemotherapy and attached to the Medical Directorate of the Registry of Bone Marrow Donors (REDMO) of the Foundation).
Become a member of the cure for leukaemia!


