Chronic myeloid leukaemia
Before the discovery of tyrosine kinase inhibitors (TKIs), chronic myeloid leukaemia patients had a dismal prognosis. Today, the extraordinary development of these therapies has led to 90% of patients seeing their disease become chronic with a relatively normal quality of life.
The information provided on www.fcarreras.org is intended to support, not replace, the relationship that exists between patients/visitors to this website and their physician.

Carlos
Chronic myeloid leukemia.
“I was diagnosed with chronic myeloid leukaemia in 2011. Since then, I live with the disease and the medication. I do sports challenges to benefit the Josep Carreras Foundation when I feel well. Being UNSTOPPABLE means not giving up, fighting every day, and maintaining human values to help others.”
Information endorsed by… ![]()
Information provided by Dr. Valentín García Gutiérrez. Haematology and Haemotherapy Service; Ramón y Cajal University Hospital; Ramón y Cajal Institute of Health Research; University of Alcalá. Madrid Medical Association (Co. 2859316).
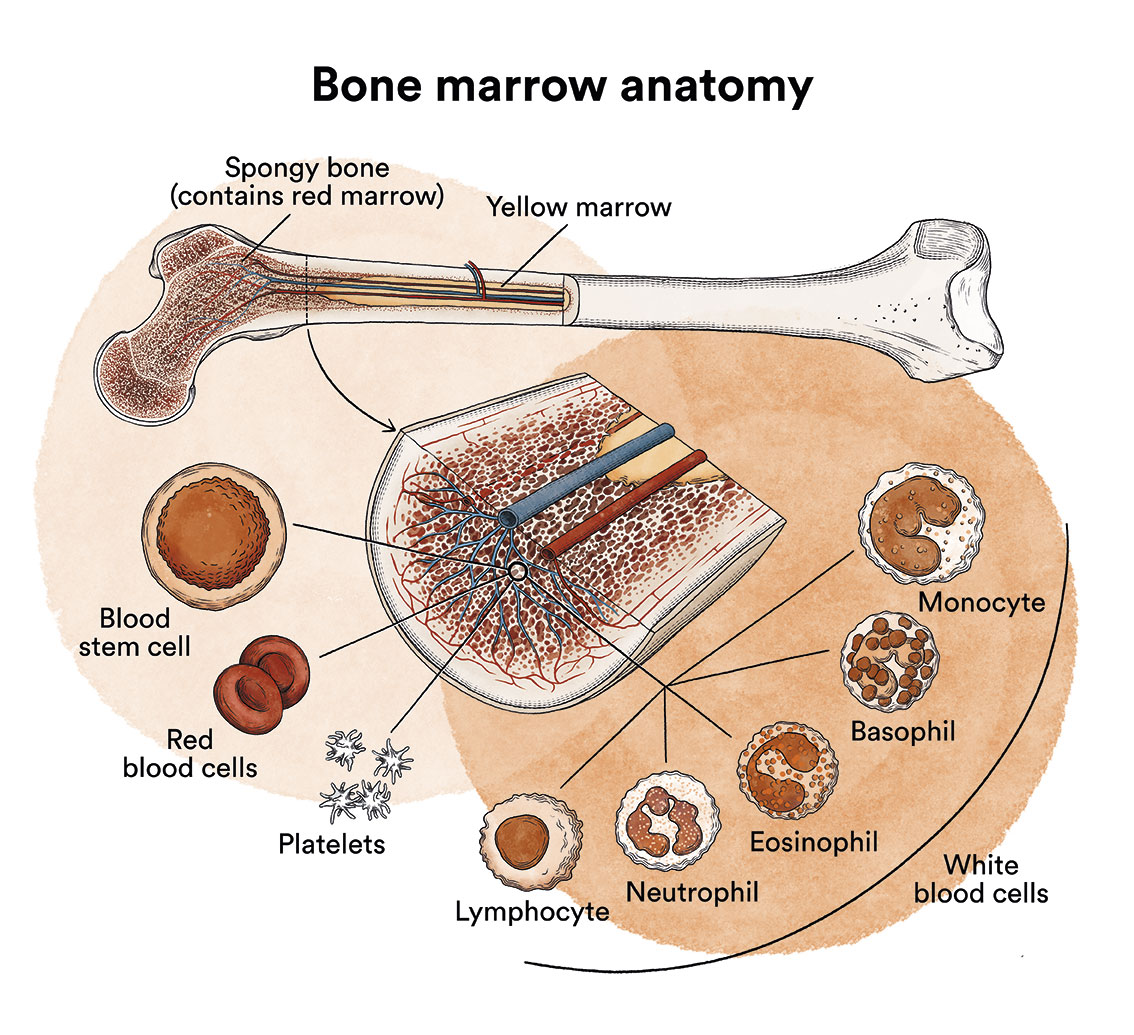
How does bone marrow work and what are the types of blood cells?
Chronic myeloid leukaemia (CML) is a type of blood cell cancer that originates in the bone marrow. See section Leukaemia, bone marrow and blood cells.
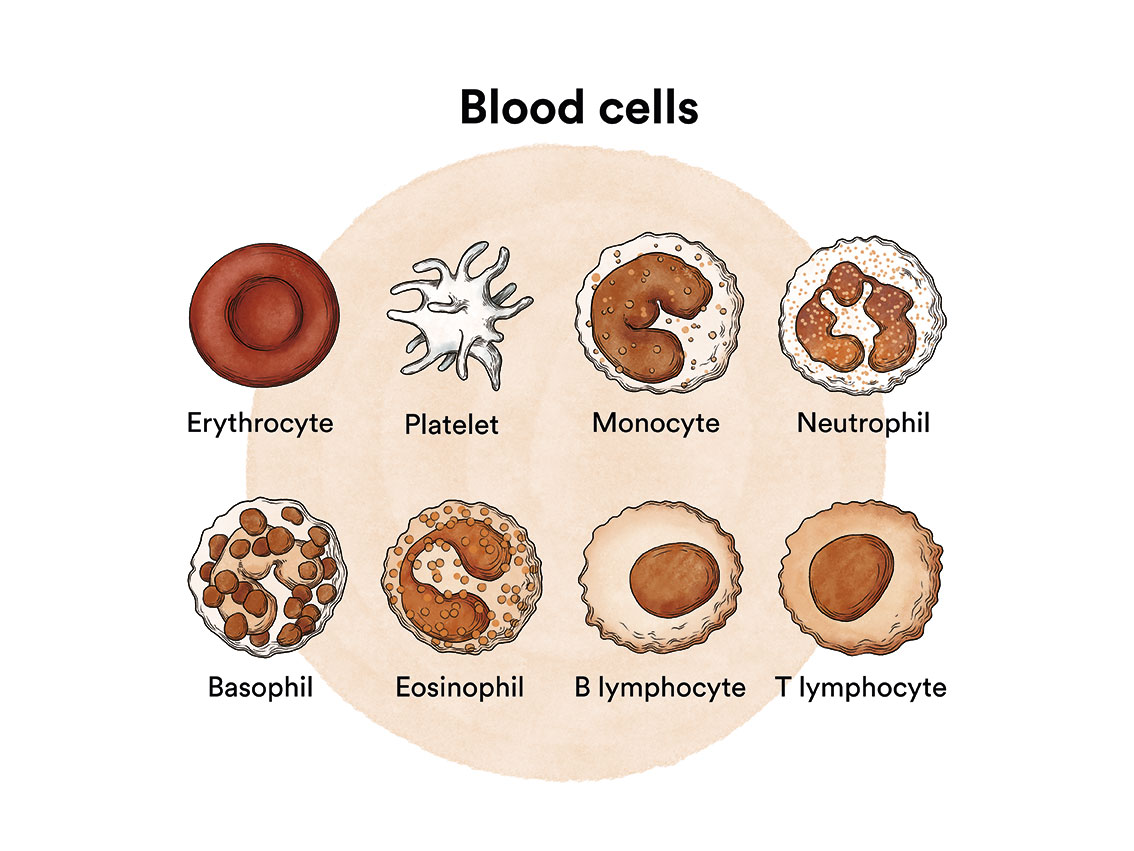
What is chronic myeloid leukaemia and who does it affect?
Chronic myeloid leukaemia (CML) is a cancerous disease of the bone marrow characterised by an uncontrolled increase in the production of some of the cells it produces.
CML accounts for about 20% of all leukaemias, although it should be considered a rare disease, affecting approximately 1 case per 100,000 population. The average age of onset is 56 years, with very infrequent occurrence in children. Due to the significant increase in survival rates over recent decades, the prevalence of CML has increased considerably in recent years.
The disease falls within a group of diseases called chronic myeloproliferative neoplasms (MPN), which share the common characteristic of being of slow progression.
Diagnosis usually occurs in an asymptomatic phase which non-aggressive known as chronic phase. Until the advent of current drugs (tyrosine kinase inhibitors – TKIs), the natural progression of the disease was to remain in the chronic phase for approximately 3 to 5 years, to then progress into an accelerated phase and then to a blastic phase. These accelerated or blastic phases are considered to be the most advanced stages of the disease, they are very similar to acute leukaemia, and therefore have a poor prognosis. Fortunately, thanks to currently available treatments, the likelihood of progression is less than 10%.
It is important to differentiate between chronic myelomonocytic leukaemia (CMML) and chronic myeloid leukaemia which, despite their similar names, are completely different entities.

Josep
Chronic myeloid leukaemia.
“In 2015 I was diagnosed with chronic myeloid leukaemia. Thanks to the support of my family and friends, doctors, and a treatment that has evolved a lot in recent years, today I have my disease under control and I can lead a normal life.”
What are the causes of chronic myeloid leukaemia?
It is not known why chronic myeloid leukaemia develops, although in some cases it has been attributed to ionising radiation, but in most cases, it is an acquired disease related to ageing.
Chronic myeloid leukaemia, like other cancers, is not contagious. See section Leukaemia, bone marrow and blood cells.
At a cytogenetic level, CML is caused by the genetic mutation of an oncogene called BCR-ABL. This alteration is detected in chromosome analysis because it is the result of the translocation (exchange) of two chromosomes, 22 and 9. The result of this exchange is an abnormal chromosome called Philadelphia (Ph+) or t (9,22). This Philadelphia chromosome is an alteration that is only found in blood cells. It is an acquired (not congenital) disorder and therefore is neither inherited nor can it be passed on to children.
What are the symptoms of chronic myeloid leukaemia?
In the early stages of chronic myeloid leukaemia, patients often do not feel any discomfort. The most frequent form of presentation of this disease is the chance finding of an analytical abnormality. Around 30% of patients may suffer from general tiredness, weight loss, sweating, bone pain, bleeding, repetitive infections or abdominal discomfort due to an increase in the size of the spleen (splenomegaly).
How is chronic myeloid leukaemia diagnosed?
It is not possible to confirm the diagnosis of chronic myeloid leukaemia (CML) with a blood test alone. After suspicion of the disease based on blood test abnormalities, which usually consist of a marked increase in leukocytes (white blood cells) -leukocytosis- and/or platelets, and the leukocyte counts at which CML patients are diagnosed can range from as low as 15,000/mm3 to over 500,000/mm3, a thorough examination of the patient’s blood and bone marrow should be performed to confirm the disease. The diagnosis will be confirmed after the presence of the Philadelphia chromosome has been detected. The study of this chromosome is usually performed on a bone marrow aspirate (obtained through bone marrow puncture).
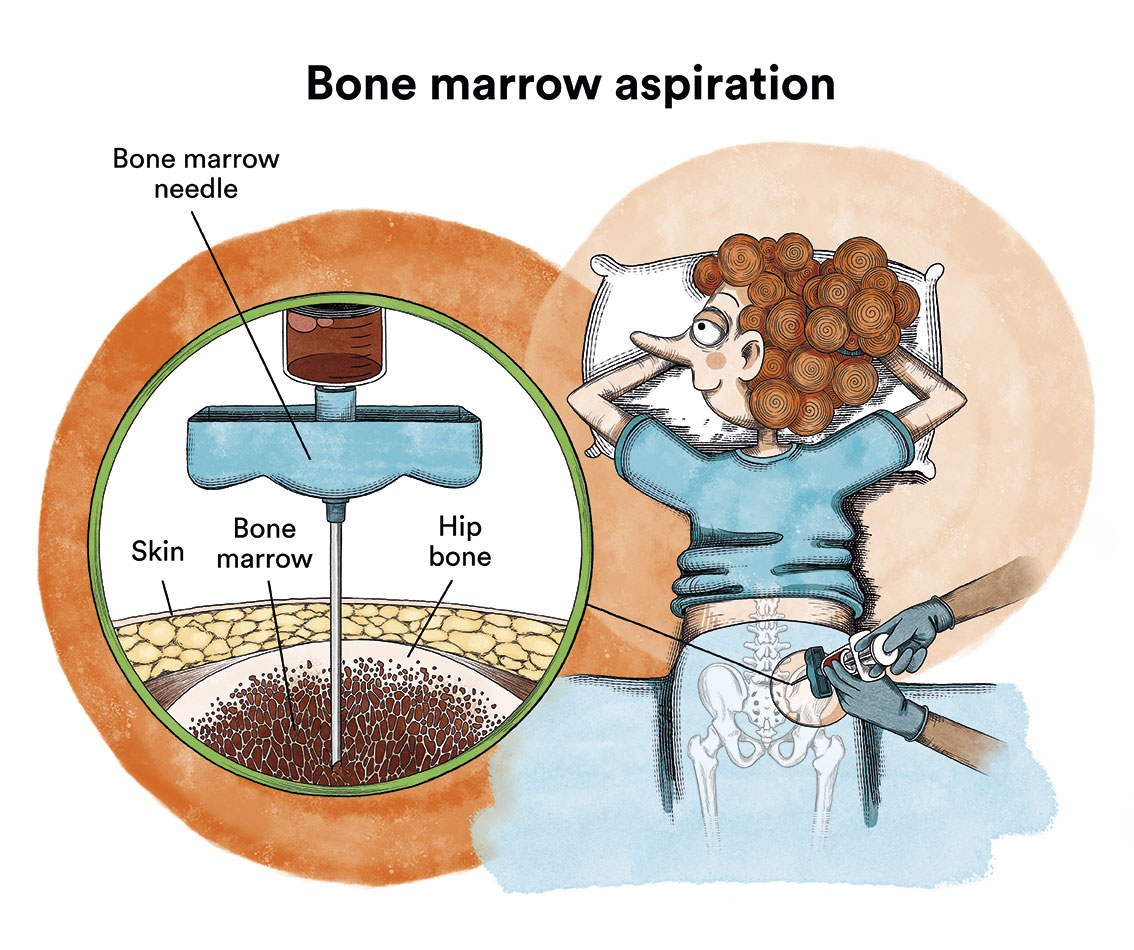
As a complementary diagnostic test, which will later serve to assess the response to treatment, the BCR-ABL oncogene should be found in blood by means of conventional blood sampling.
In most patients, CML is diagnosed when it is in the so-called chronic phase (95%). At this stage, patients have few or no symptoms and the number of immature cells or blasts is less than 10%. In the blastic phase, blood or bone marrow tests show an increase in immature cells in excess of 20% or the appearance of these cells in other parts of the body. In this phase, more severe symptoms, anaemia and an increasing number of white blood cells appear despite adjusting treatment.
What is the treatment for chronic myeloid leukaemia?
Current treatment for CML is by administering drugs called tyrosine kinase inhibitors (TKIs), a treatment directed against the BCR-ABL protein, which, in principle, should be taken indefinitely. All TKIs are oral drugs with a very good toxicity profile.
Imatinib (2001) was the first drug approved for this pathology, demonstrating high efficacy with an excellent safety profile. Nilotinib, dasatinib and bosutinib are the so-called “second generation” TKIs. Ponatinib is often considered a “third generation” inhibitor. When the TKIs cause the Ph chromosome to disappear from the bone marrow, this is referred to a complete cytogenetic response (CCR). When, after a longer period of time, the BCR-ABL gene disappears from the blood and marrow, we refer to a complete molecular response (CMR). Tyrosine kinase inhibitors are oral drugs.
Nilotinib and dasatinib are indicated for both newly diagnosed patients and for patients who fail to achieve adequate control after treatment with imatinib (which may occur in up to 40% of patients). Bosutinib and ponatinib are approved for patients who do not respond adequately to a second-generation TKI. A small percentage of patients may develop a specific mutation called T315I that confers resistance to both imatinib and second-generation TKIs, in which case treatment with ponatinib is essential.
The market in Spain currently offers a generic imatinib, which is manufactured by various pharmaceutical companies. All of these have demonstrated an adequate bioequivalence profile with the original brand (Glivec®), yet at a much lower price. Since early 2017, almost all patients treated with imatinib have been treated with one of the available generic drugs.
Bone marrow transplantation, which for years was considered the treatment of choice for most CML patients, is now reserved for patients who fail to respond to treatment with the various TKIs, which occurs in less than 10% of cases.
Allogeneic bone marrow transplantation is life-threatening and is reserved for those few patients who fail to respond to treatment with TKI.
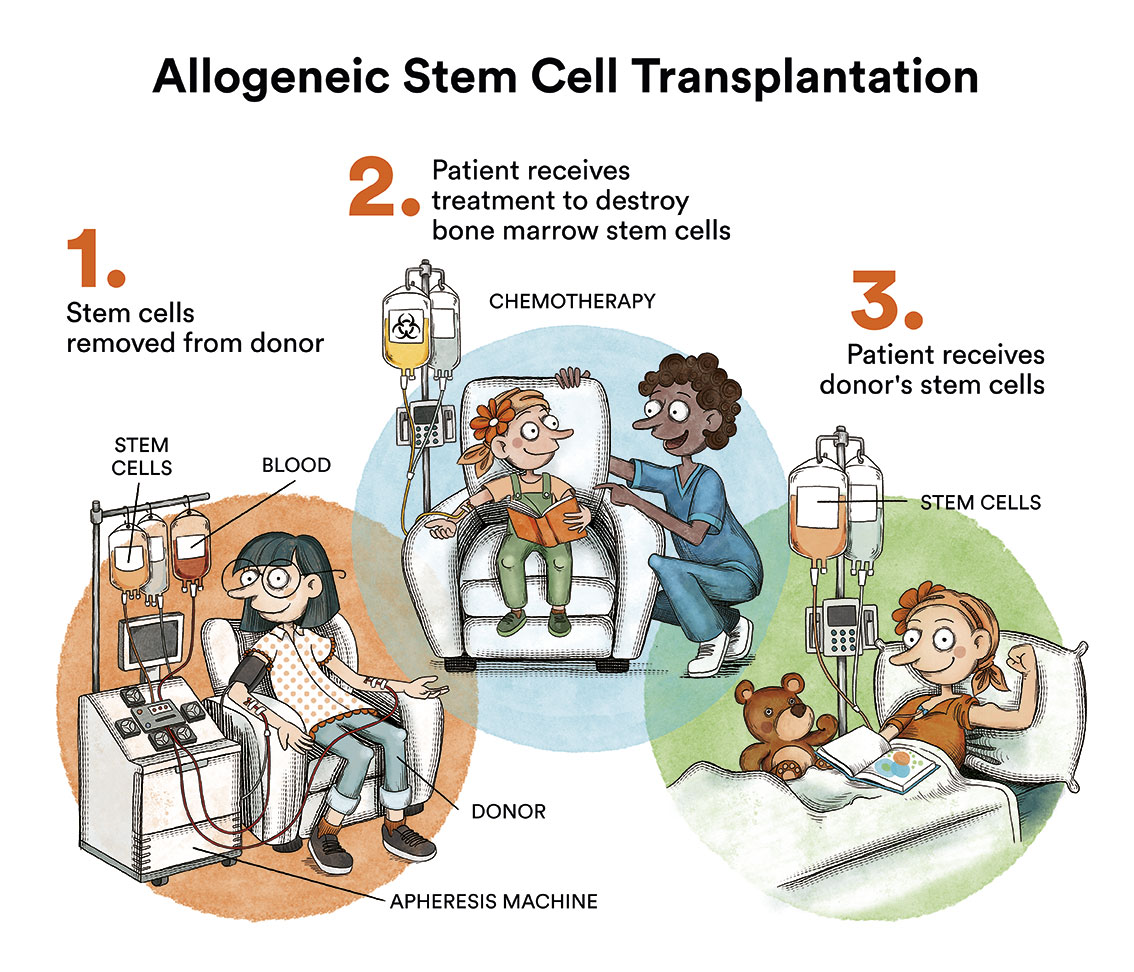
TKIs are generally well-tolerated drugs. In the first months of the disease, patients often suffer from mild side effects common to all of them, which are usually transient. The most common side effects are nausea, fluid retention, abdominal discomfort or cramps. It is important for the patient to be aware that some of the side effects may be asymptomatic and therefore only detectable by means of blood tests, therefore close monitoring of the disease is essential. Most side effects can be adequately managed with reductions/interruptions of the medication, and it may be necessary to add some complementary treatment on an ad hoc basis. It is essential that patients discuss any side effects with their referring physician and do not make any medication changes at their own initiative. There are other drug-specific side effects whose frequency may be conditional upon certain characteristics of the individual patient, thus the choice of treatment should be individualised and adapted to each particular patient.
Despite their excellent safety profile, TKIs may interact with other drugs that patients might need for treating other conditions. It is important to consult with your physician before starting a new medication.
Although TKIs achieve excellent disease control in the vast majority of patients, the general indication is to maintain treatment indefinitely. However, there is already evidence of how very select patients (due to achieving a high quality and sustained molecular response over time), after several years of treatment, could safely discontinue treatment. This possibility of treatment interruption is even included in the data sheet for nilotinib. However, it is crucial for patients to be aware that discontinuation should only be considered for selected patients, under close monitoring and in reference centres.
What is the prognosis for patients with chronic myeloid leukaemia?
The prognosis of CML has changed dramatically in recent years. Since the advent of TKIs, CML has gone from a life expectancy of only 5 years in patients who are not candidates for bone marrow transplantation to a life expectancy similar to that of the general population in those patients diagnosed in the chronic phase (who constitute the vast majority of CML patients). Thus, today, the overall survival of the disease is around 90%, with most deaths being unrelated to the disease.
However, there are some circumstances (the most decisive being the stage of the disease) that would condition the prognosis of the disease, which should be evaluated at diagnosis by your physician in order to choose the most appropriate treatment.
Pregnancy and other special situations
Given the excellent prognosis of the disease and the fact that approximately 20% of cases diagnosed in CML will be diagnosed under the age of 40, the desire for conception is frequently raised in this disease.

According to the data sheet of the TKIs, their teratogenic effect (risk of malformations) is unknown, and it is recommended to avoid taking these drugs during and before pregnancy. However, the increasing experience acquired in this field is tending towards supporting the possibility of TKI treatment in males without being associated with such teratogenic effects.
There is now sufficient evidence of patients with CML who have gone on to have a normal pregnancy following appropriate disease management.
It is imperative that the patient raises the situation in an experienced centre where the various possible options will be considered. These options may include continuation of the drug (for males only), discontinuation (temporarily or permanently) in patients with an adequate response, or replacement with another drug such as interferon.
We advise CML patients in Spain to contact AELEMIC, the Spanish association of patients with chronic myeloid leukaemia.
Links of interest concerning medical issues relating to chronic myeloid leukaemia
- Acute lymphoblastic leukaemia in adults. American Cancer Society.
- Adult Acute Lymphoblastic Leukemia Treatment. National Cancer Institute
- Acute Lymphoblastic Leukemia. Leukemia & Lymphoma Society
- Acute lymphoblastic leukaemia. Bloodwise UK
Links of interest concerning medical issues relating to chronic myeloid leukaemia
TESTIMONIAL MATERIALS
You can order the booklets in paper format for free delivery in Spain by e-mail: imparables@fcarreras.es
BONE MARROW TRANSPLANT
- Bone Marrow Transplant Guide. Josep Carreras Foundation (content in Spanish)
- What is HLA and how does it work? Josep Carreras Foundation (content in Spanish)
- Graft-versus-Host Disease. Josep Carreras Foundation (content in Spanish)
- History of Bone Marrow Transplantation. Josep Carreras Foundation (content in Spanish)
- How is the search for an anonymous donor conducted? Josep Carreras Foundation (content in Spanish)
FOOD
- How to maintain a healthy diet during treatment? Josep Carreras Foundation (content in Spanish)
- Nutrition guide. Leukemia & Lymphoma Society
OTHER
- Ideas on what to take with me to the isolation chamber. Josep Carreras Leukaemia Foundation (content in Spanish)
- Travel tips for people with cancer. Josep Carreras Leukaemia Foundation (content in Spanish)
- Physiotherapy manual for haematological and transplant patients. Josep Carreras Leukaemia Foundation (content in Spanish)
- Prevention and treatment of oral mucositis. Josep Carreras Leukaemia Foundation (content in Spanish)
- Oral hygiene in oncohaematological patients. Josep Carreras Leukaemia Foundation (content in Spanish)
- Fertility manual: Suffering from blood cancer and becoming a parent. Josep Carreras Leukaemia Foundation (content in Spanish)
- Skin care in the oncohaematological patient. Josep Carreras Leukaemia Foundation (content in Spanish)
- Aesthetic Oncology Manual. Josep Carreras Leukaemia Foundation (content in Spanish)
- Leukaemia and sexuality. Josep Carreras Leukaemia Foundation (content in Spanish)
- 7 ways to wear a scarf. Josep Carreras Leukaemia Foundation (content in Spanish)
Links of interest: local/provincial or state entities that can provide you with resources and services specialised in leukaemia or cancer patients:
In Spain there is a large network of associations for haematological cancer patients that, in many cases, can inform you, advise you and even carry out certain procedures. These are the contacts of some of them by Autonomous Communities:
All these organisations are external to the Josep Carreras Foundation.
STATE
AELEMIC (Asociación Española de Pacientes de Leucemia Mieloide Crónica)
AEAL (ASOCIACIÓN ESPAÑOLA DE AFECTADOS POR LINFOMA, MIELOMA y LEUCEMIA)
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact with the nearest branch or call 900 100 036 (24h).
AELCLES (Agrupación Española contra la Leucemia y Enfermedades de la Sangre)
Josep Carreras Leukaemia Foundation
GEPAC (GRUPO ESPAÑOL DE PACIENTES CON CÁNCER)
ANDALUCÍA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
ALUSVI (ASOCIACIÓN LUCHA Y SONRÍE POR LA VIDA). Sevilla
APOLEU (ASOCIACIÓN DE APOYO A PACIENTES Y FAMILIARES DE LEUCEMIA). Cádiz
ARAGÓN
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
ASPHER (ASOCIACIÓN DE PACIENTES DE ENFERMEDADES HEMATOLÓGICAS RARAS DE ARAGÓN)
ASTURIAS
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
ASTHEHA (ASOCIACIÓN DE TRASPLANTADOS HEMATOPOYÉTICOS Y ENFERMOS HEMATOLÓGICOS DE ASTURIAS)
CANTABRIA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
CASTILLA LA MANCHA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
CASTILLA LEÓN
ABACES (ASOCIACIÓN BERCIANA DE AYUDA CONTRA LAS ENFERMEDADES DE LA SANGRE)
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
ALCLES (ASOCIACIÓN LEONESA CON LAS ENFERMEDADES DE LA SANGRE). León.
ASCOL (ASOCIACIÓN CONTRA LA LEUCEMIA Y ENFERMEDADES DE LA SANGRE). Salamanca.
CATALUÑA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
ASSOCIACIÓ FÈNIX. Solsona
FECEC (FEDERACIÓ CATALANA D’ENTITATS CONTRA EL CÁNCER
FUNDACIÓ KÁLIDA. Barcelona
FUNDACIÓ ROSES CONTRA EL CÀNCER. Roses
LLIGA CONTRA EL CÀNCER COMARQUES DE TARRAGONA I TERRES DE L’EBRE. Tarragona
ONCOLLIGA BARCELONA. Barcelona
ONCOLLIGA GIRONA. Girona
ONCOLLIGA COMARQUES DE LLEIDA. Lleida
ONCOVALLÈS. Vallès Oriental
OSONA CONTRA EL CÀNCER. Osona
SUPORT I COMPANYIA. Barcelona
VILASSAR DE DALT CONTRA EL CÀNCER. Vilassar de Dalt
VALENCIAN COMMUNITY
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
ASLEUVAL (ASOCIACIÓN DE PACIENTES DE LEUCEMIA, LINFOMA, MIELOMA Y OTRAS ENFERMEDADES DE LA SANGRE DE VALENCIA)
EXTREMADURA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
AFAL (AYUDA A FAMILIAS AFECTADAS DE LEUCEMIAS, LINFOMAS; MIELOMAS Y APLASIAS)
AOEX (ASOCIACIÓN ONCOLÓGICA EXTREMEÑA)
GALICIA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
ASOTRAME (ASOCIACIÓN GALLEGA DE AFECTADOS POR TRASPLANTES MEDULARES)
BALEARIC ISLANDS
ADAA (ASSOCIACIÓ D’AJUDA A L’ACOMPANYAMENT DEL MALALT DE LES ILLES BALEARS)
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
CANARY ISLANDS
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
AFOL (ASOCIACIÓN DE FAMILIAS ONCOHEMATOLÓGICAS DE LANZAROTE)
LA RIOJA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
MADRID
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
AEAL (ASOCIACIÓN ESPAÑOLA DE LEUCEMIA Y LINFOMA)
MURCIA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
NAVARRA
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
BASQUE COUNTRY
AECC (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER). Present is the different provinces and in many municipalities. Contact the nearest branch.
PAUSOZ-PAUSO. Bilbao
AUTONOMOUS CITIES OF CEUTA AND MELILLA
AECC CEUTA (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER)
AECC MELILLA (ASOCIACIÓN ESPAÑOLA CONTRA EL CÁNCER)
Support and assistance
We also invite you to follow us through our main social media (Facebook, Twitter and Instagram) where we often share testimonies of overcoming this disease.
If you live in Spain, you can also contact us by sending an e-mail to imparables@fcarreras.es so that we can help you get in touch with other people who have overcome this disease.
* In accordance with Law 34/2002 on Information Society Services and Electronic Commerce (LSSICE), the Josep Carreras Leukemia Foundation informs that all medical information available on www.fcarreras.org has been reviewed and accredited by Dr. Enric Carreras Pons, Member No. 9438, Barcelona, Doctor in Medicine and Surgery, Specialist in Internal Medicine, Specialist in Hematology and Hemotherapy and Senior Consultant of the Foundation; and by Dr. Rocío Parody Porras, Member No. 35205, Barcelona, Doctor in Medicine and Surgery, Specialist in Hematology and Hemotherapy and attached to the Medical Directorate of the Registry of Bone Marrow Donors (REDMO) of the Foundation).
Become a member of the cure for leukaemia!


