Childhood acute lymphoblastic leukaemia MLL-AF4
Leukaemia is the most common cancer among children. It affects more than 350 children a year in our country, a third of which are under 4 years of age. Leukaemia accounts for 30% of all paediatric cancers, with B-acute lymphoblastic leukaemia being the most common form among children (80% of cases). MLL-AF4 acute lymphoblastic leukaemia especially affects infants under one year of age.
The information provided on www.fcarreras.org is intended to support, not replace, the relationship that exists between patients/visitors to this website and their physician.
Information provided by Dr. Pablo Menéndez. Researcher specialising in B-ALL MLLAF4 at the Josep Carreras Leukaemia Research Institute.

Abril
Acute myeloid leukemia.
“When I was five months old, I was diagnosed with acute lymphoblastic leukaemia. After undergoing a very hard treatment, I received a blood stem cell transplant from an anonymous donor finded by the REDMO program of the Josep Carreras Foundation. After 4 years of recovery, and when I was already well, the leukemia returned. At that time, I was lucky enough to be able to undergo a CART immunotherapy clinical trial, the first to be done in Spain with children. It was 2016. Since then, I have been fine. I went back to school, to dancing, to music, to reading… and since then I have enjoyed life very much. I am unstoppable.”
What is leukaemia, bone marrow and what are the types of blood cells?
Leukaemia is a type of blood cell and bone marrow cancer. See section Leukaemia, bone marrow and blood cells.
What is childhood acute lymphoblastic leukaemia MLL-AF4?
Acute lymphoblastic leukaemia (ALL) is a type of cancer in which the bone marrow produces too many immature lymphocytes (a type of white blood cell), known as lymphoblasts. These cells prevent the growth of other blood cells. ALL is the most common cancer in children, and most diagnoses are B-acute lymphoblastic leukaemia (80% of leukaemia cases in children).
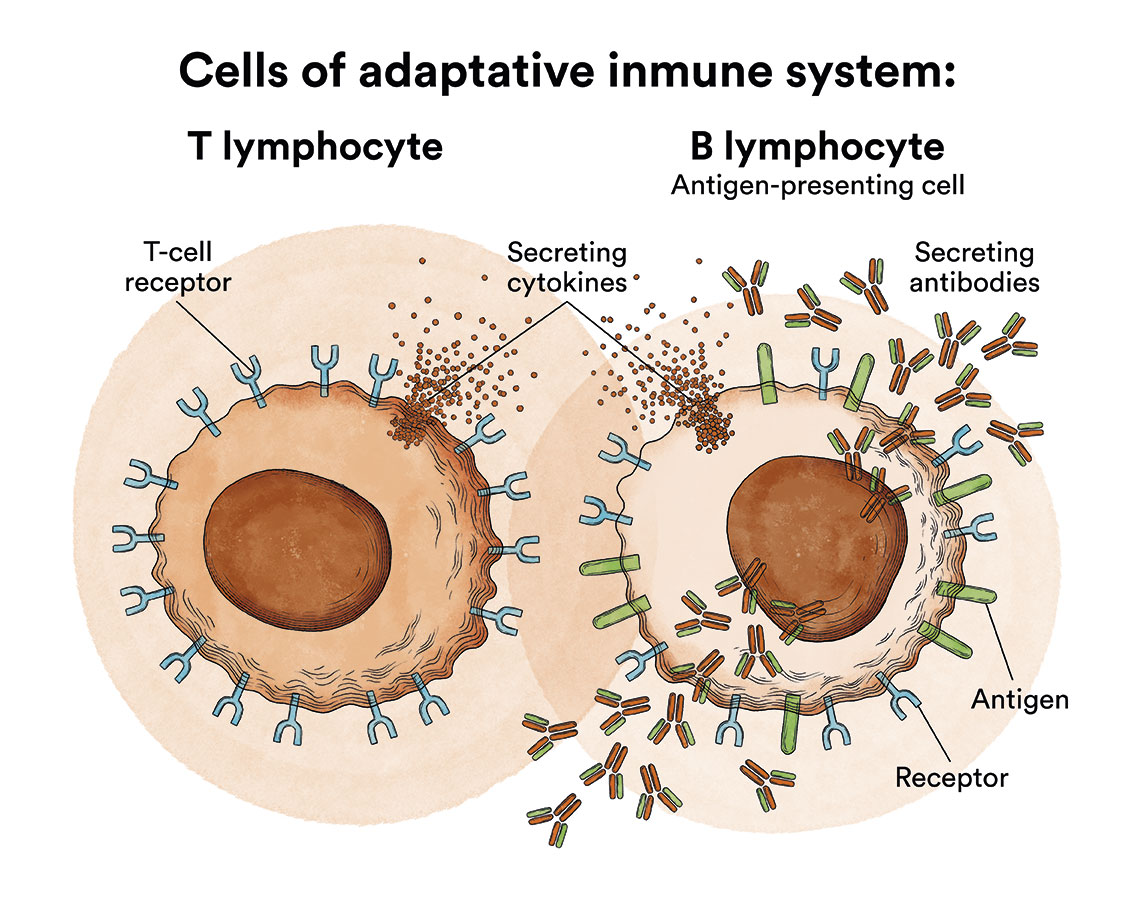
Lymphocytes are a type of leukocyte or white blood cell and can be of two types, B and T. Depending on whether the lymphoblasts originate from B or T cells, ALL will be referred to as B-ALL or T-ALL.
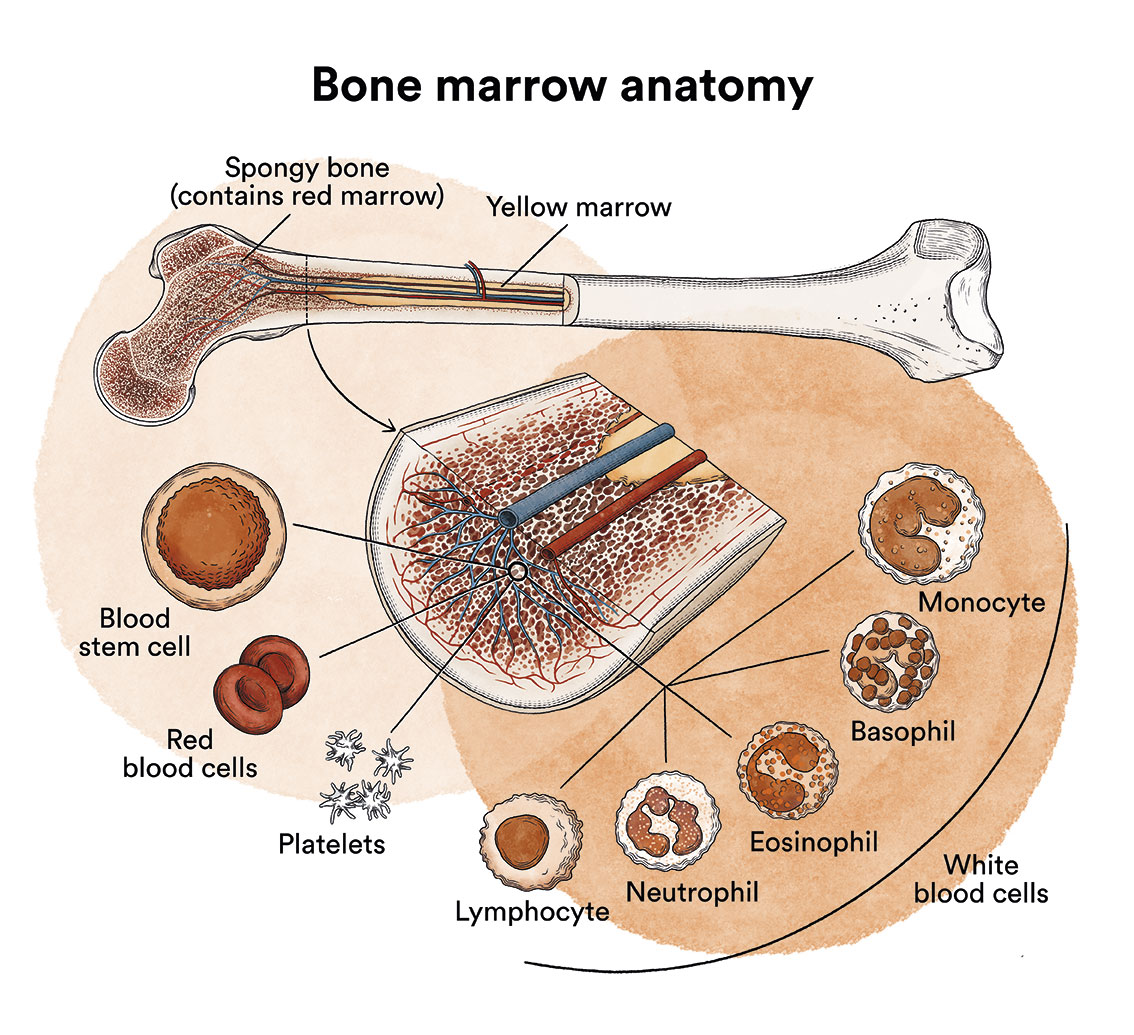
As we have seen in ‘Leukaemia, bone marrow and blood cells’, the bone marrow produces blood stem cells (immature cells) that will eventually develop into mature blood cells. A blood stem cell becomes a myeloid stem cell or a lymphoid stem cell.
A myeloid stem cell develops into one of the three types of mature blood cells:
- Red blood cells, which carry oxygen to other tissues and organs in the body.
- Granulocytes, white blood cells that help fight infection and other diseases.
- Platelets, which help blood to clot when a blood vessel ruptures.
A lymphoid stem cell develops into a lymphoblast and later into one of the three types of lymphocytes (white blood cells):
- B lymphocytes, which produce antibodies to help fight infections in the body.
- T lymphocytes, which help B lymphocytes produce antibodies to fight infection.
- Natural cytolytic lymphocytes or NK lymphocytes (natural killer), a type of immune cell that contains enzymes which can destroy tumour cells or virus-infected cells.
In children affected by B-acute lymphoblastic leukaemia, there are too many stem cells that transform into B lymphoblasts.
Acute lymphoblastic leukaemias affecting infants (infants less than 1 year old) are biologically different from ALL in older children. Infantile ALL with t(4;11) MLL-AF4+ rearrangement is a particularly aggressive subtype of leukaemia which often develops before the first year of life. Most babies (about 80%) have this gene rearrangement called MLL (mixed phenotype leukaemia). The MLL-AF4 translocation is the characteristic genetic marker of a particularly aggressive B-acute lymphoblastic leukaemia (B-ALL), which commonly affects children under one year of age and has a low response to chemotherapy, reducing survival rates for this disease to less than 30%. It is a type of leukaemia that affects fewer than 10 babies a year in Spain.
Paolo
Acute lymphoblastic leukaemia.
“In 2019, when he had 6 months old, Paolo was diagnosed with MLL+ acute lymphoblastic leukaemia. The only option to cure Paolo was undergoing a bone marrow transplant. There we begin a path full of uncertainties, fear, and pain. Our life as new parents was not what we had dreamed of and our lives changed completely. After 8 months of treatment, the day arrived that we entered that isolated room so that Paolo could receive the stem cells thanks to an anonymous mother who had donated the umbilical cord of her baby. I find it hard to believe that time has passed after that day when I felt like our clock stopped. Today Paolo is growing healthy and strong with his little brother thanks to the research and work of the medical team and the Josep Carreras Foundation. We celebrate that Paolo is with us. Today we celebrate LIFE every day.”
What are the causes of MLL-AF4 acute lymphoblastic leukaemia?
The exact cause of B-ALL is unknown. Research in this field is important and the genetic changes that occur in leukaemic cells are becoming known. However, in most children with B-ALL, the triggers or factors that led to the leukaemia are unknown.
It is currently uncertain whether the MLL-AF4 fusion in this leukaemia is capable of generating the disease “on its own” or whether it requires the presence of a second mutation to initiate the leukaemic process. The cells involved in this type of leukaemia appear to become cancerous before birth, which means that this type of leukaemia is not simply a case of blood cells multiplying incorrectly but a problem of early foetal development in the first place. This makes it a different disease from B-ALL in adults or even in other children.
The prenatal origin of the t(4;11) translocation has been demonstrated by clonogenicity studies in monozygotic twins, in which both have the translocation with the same breakpoint (Ford et al. 1993; Gale et al. 1997), and by retrospective studies in which the same translocation detected at diagnosis in the patient was present in samples collected at birth (in the so-called “heel prick” test for metabolopathies) (Greaves 2002). These data suggest that the target cell in pro-B ALL could be a cell of embryonic origin or an early developing haematopoietic cell, which would explain the presence of the fusion gene at birth.
Leukaemia, like other cancers, is not contagious. See section Leukaemia, bone marrow and blood cells.
What are the symptoms of B-acute lymphoblastic leukaemia?
In B-acute lymphoblastic leukaemia, the production of normal blood cells is disrupted by the growth of leukaemic cells in the bone marrow. This can lead to:
- Tiredness and paleness (due to anaemia).
- Appearance of purple and small pink patches on the skin (petechiae) or other bleeding due to low platelet count.
- Fever and infections that do not progress well (due to leukocyte malfunction).
- Joint and bone pain (due to leukaemia cells having invaded the bone marrow).
- Adenopathies or enlarged lymph nodes (due to the occupancy of leukaemic cells in the lymphatic system).
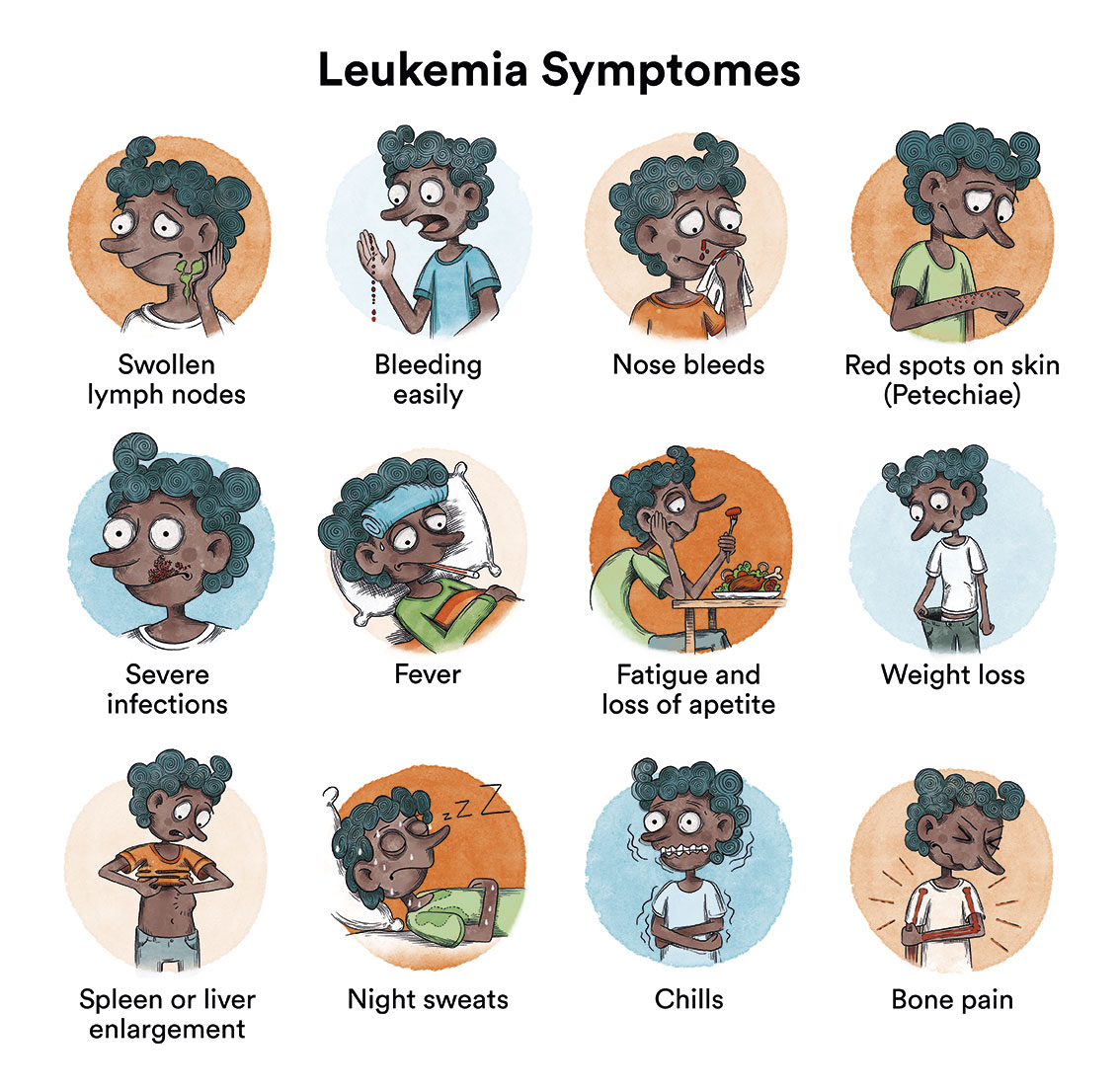
At the onset of the disease, all these symptoms can be very similar to those of a virus infection. When symptoms continue for more than 2-4 weeks, a diagnosis can be obtained in most cases. As these symptoms are not specific or exclusive to leukaemia, it is very common for the patient to have visited a doctor several times before being diagnosed. Generally, this does not influence the child’s curative options. It should be noted that MF4 ALL has a very short latency (appearing before the first year of life),
How is MLL-AF4 B-acute lymphoblastic leukaemia diagnosed?
Generally, if a baby presents the characteristic symptoms, leukaemia can be diagnosed with a blood test. When the blood is examined under a microscope, leukaemic cells will be visible. Sometimes, at the beginning of the disease, these cells are not seen in the blood and leukaemia is suspected due to the symptoms and some alterations in the blood tests. The diagnosis is confirmed by a bone marrow puncture (iliac crests) and analysis of the cells obtained.
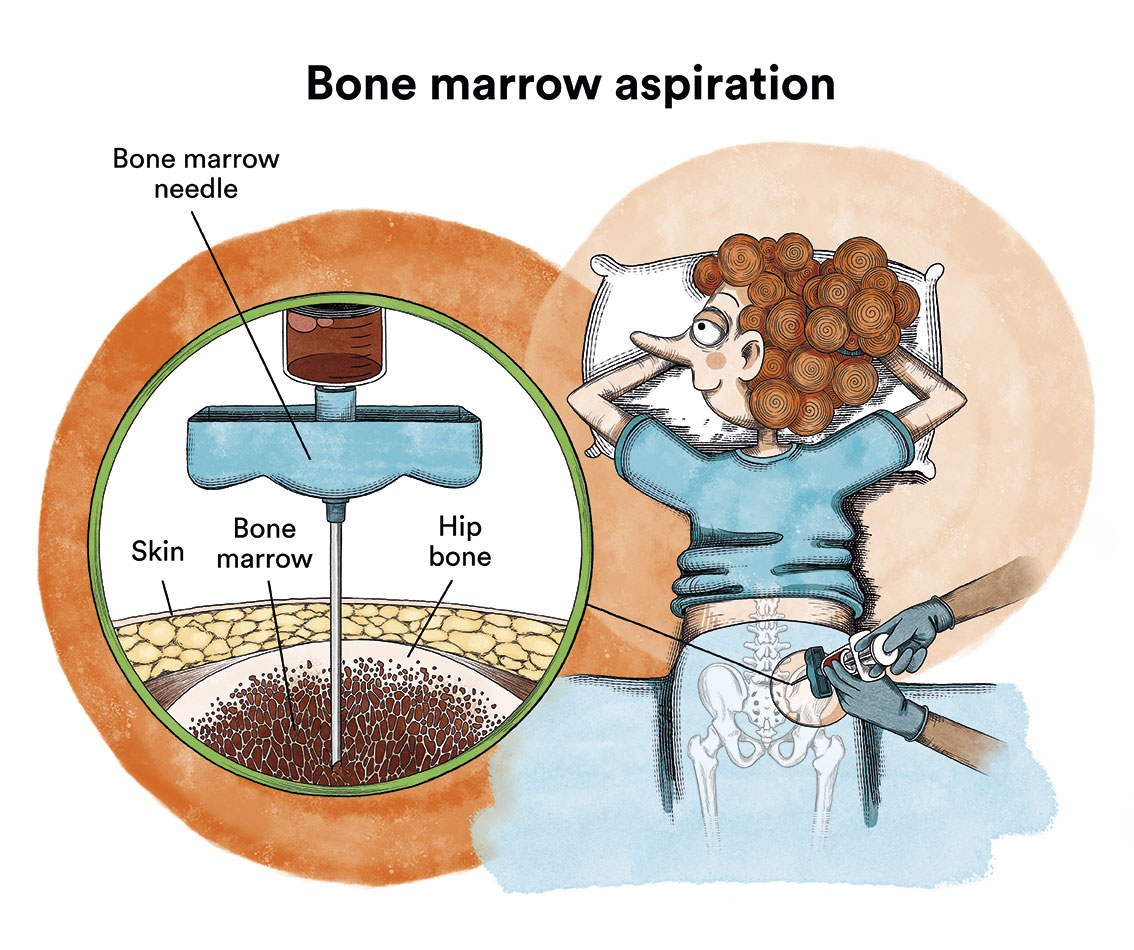
What is the treatment for MLL-AF4 B-acute lymphoblastic leukaemia in infants?
 The goal of treatment for acute lymphoblastic leukaemia is to eliminate the leukaemic cells to allow the bone marrow to return to its normal functioning.
The goal of treatment for acute lymphoblastic leukaemia is to eliminate the leukaemic cells to allow the bone marrow to return to its normal functioning.
Basically, chemotherapy with high-dose cytarabine is the main treatment that infants with ALL will receive. It involves the use of drugs that kill cancer cells by preventing them from reproducing. The treatment has different phases and normally lasts about 2 years. In recent years, the application of the INTERFANT-99 protocol to these patients has managed to increase the survival rate to approximately 30-40%, a result which, although encouraging, is still extremely adverse for more than half of the children diagnosed with this disease.

When chemotherapy is administered intravenously, to avoid repeatedly puncturing a vein, specialists implant a special device called a catheter (content in spanish). The catheter is inserted into a large vein, which allows for the administration of all types of medication, as well as for drawing blood for blood tests, thus avoiding the child from enduring repeated needle punctures.
There is a type of catheter, called a port-a-cath, which is attached to a round plastic or metal reservoir under the skin of the chest. The port-a-cath is very practical for children because it is under the skin and does not allow the infant to pull it out, it is less likely to become infected than other types of catheters and it allows the infant to bathe.
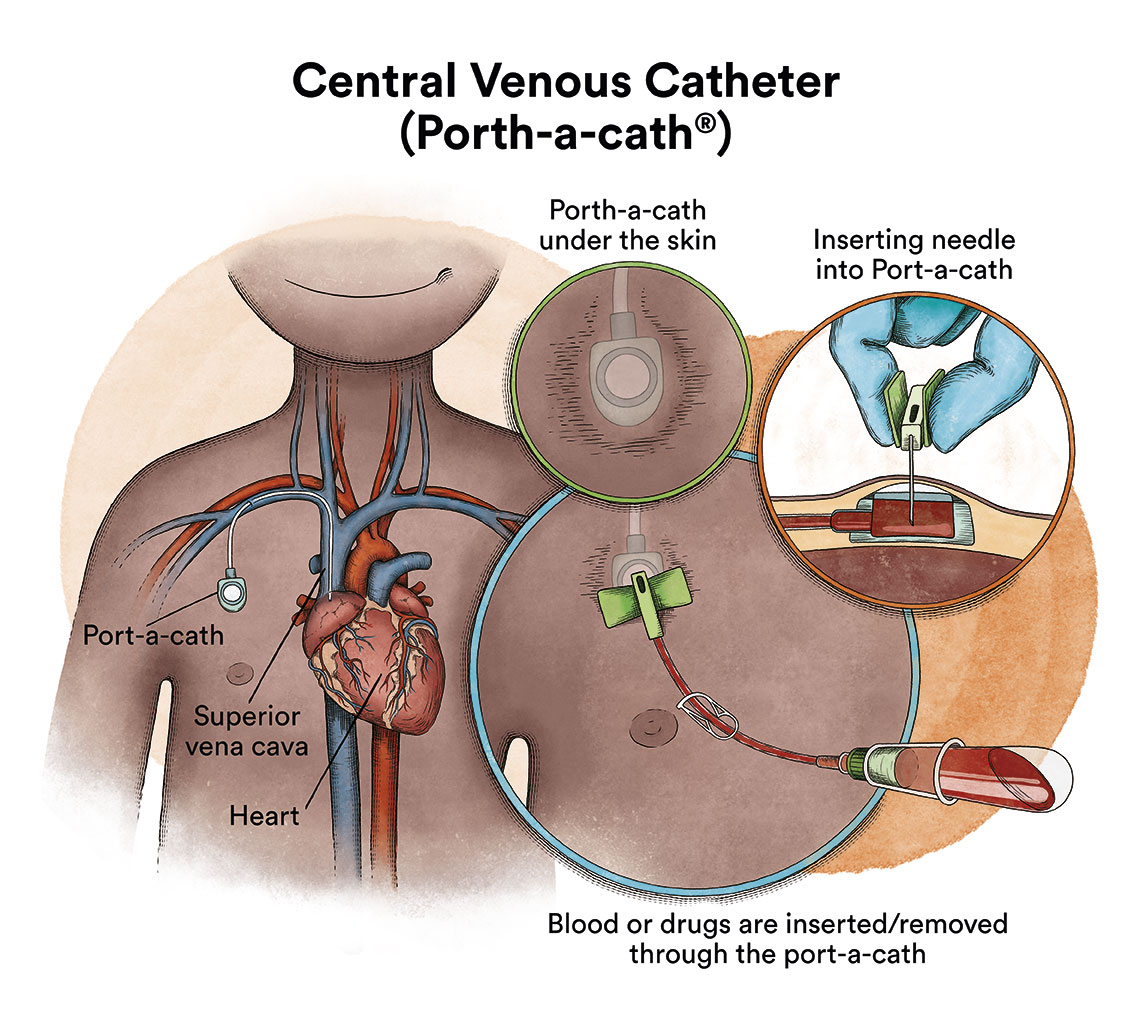
If the chemotherapy is administered through a venous catheter, it reaches almost all the cells of the body via the blood. However, most chemotherapy drugs do not fully reach the cerebrospinal fluid that bathes the brain and spinal cord. This means that there are leukaemic cells that can survive in this fluid. In order to prevent leukaemic cells that reach the cerebrospinal fluid from surviving and causing a future relapse that will affect the nervous system, chemotherapy must be administered directly into the cerebrospinal fluid, via lumbar punctures (intrathecal chemotherapy). Infants affected with MLL AF4 ALL have a high risk of central nervous system (CNS) leukaemia involvement.
MLL AF4 ALL patients are patients at high risk of relapse and will therefore receive more intensive treatment. In assigning a risk category, physicians consider:
- the presence of the MLL gene rearrangement: this rearrangement indicates that there will be a poorer response to therapy.
- age: Babies who are about 1 year old generally respond better to treatment than babies younger than 6 months.
- the number of white blood cells: a very high white blood cell count at diagnosis indicates that there will be a poorer response to therapy.
- response time to early therapy
About two-thirds of infants will have a relapse within a year of diagnosis. At present, there are no treatment protocols for relapsed ALL in children under 1 year of age. These patients should be treated by teams with specific expertise.
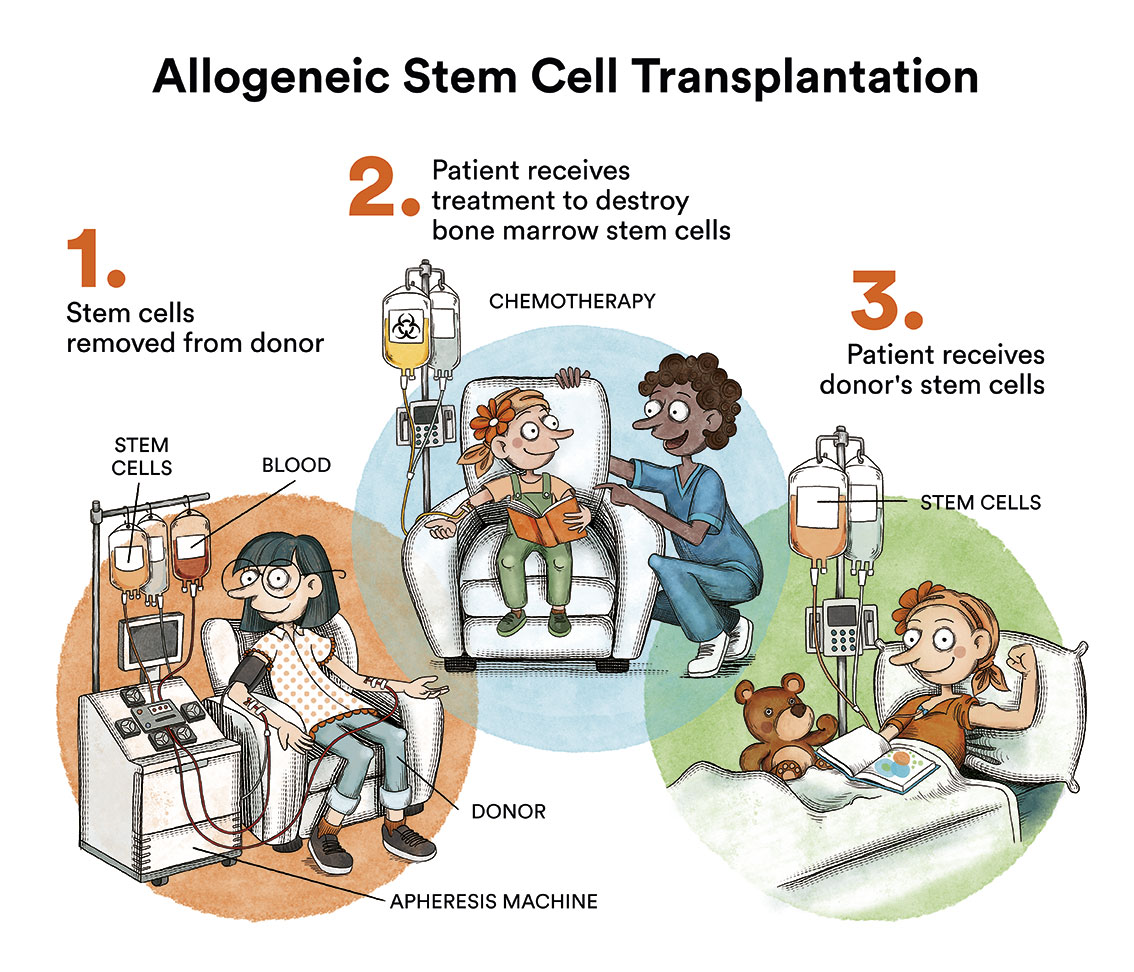
When no leukaemia cells are seen in the bone marrow or elsewhere, the patient is said to be in complete remission. In cases of relapse, chemotherapy is re-administered and, depending on the case, a bone marrow transplant is performed.
Types of chemotherapy
The chemotherapy that this type of patient will receive is high risk. In any case, all children, irrespective of the risk group into which the are classified, shall be provided with:
- an initial treatment called induction treatment, of intensive chemotherapy followed by
- a consolidation or intensification treatment. This is then followed by:
- a maintenance treatment which consists of a low intensity chemotherapy, consisting of pills (oral administration), which, in most cases, allows for the child to live an almost normal life, returning to school, sports or other extracurricular activities. For the majority of these infants, due to the high risk of relapse, a bone marrow transplantation is performed instead of the maintenance treatment.
What are the chances of children with MLL-AF4 acute lymphoblastic leukaemia being cured?
Despite a great deal of research by some teams, the overall survival rate for MF4 ALL patients is currently less than 30-40%.
Monitoring
After completing the treatment, the child will have regular check-ups performed by their haematologist and by other specialists where necessary. Monitoring is carried out to assess possible relapse and to follow-up and treat possible long-term complications. These checks are progressively spaced out until they are carried out once a year. Long-term follow-up is recommended at least annually in order for early detection and treatment of any sequelae arising from treatment or leukaemia, should they appear.
Recommendations and other practicalities
Here are some general recommendations that answer some of the most frequently asked questions by parents of children with leukaemia:
- Will their hair fall out? When? Should we cut it?
With the chemotherapy they will receive to treat the leukaemia, their hair will fall out. This usually happens 2-3 weeks after the start of chemotherapy. If the child has long hair, it is more appropriate to cut it short before it starts to fall out. It is neither necessary, nor psychologically desirable, to cut it during the first days of admission. Nor does this fact need to be explained to the child straight away. However, it is important to address this issue with the child before their hair starts to fall out. Hair regrows 2-4 weeks after starting the maintenance phase of treatment, where chemotherapy is less intense.
- Hygiene
Since the child’s defences against infection are weakened (due to the illness itself and also due to the treatment administered), it is advisable to maintain adequate hygiene of the child’s body, the hospital room and the family home, including the child’s toys.
It is advisable to avoid dusty toys and cardboard boxes. Food should also not be stored outside the refrigerator. Plants are forbidden in the room, as there are fungal spores in the soil.
Tidiness facilitates cleaning by hospital cleaning staff.
- Visits
It is advisable to reduce the number of visitors in the child’s room, as they may carry infections. It is recommended that there are no more than 2 companions in the room and that they wash their hands before entering. If any of the visitors has an infectious process (cold, conjunctivitis…) it is preferable that they do not visit.
In the case of a parent or other caregiver whose attention cannot be dispensed with, they should wear a mask and wash their hands before coming into contact with the child.
- Food
A child receiving intensive chemotherapy treatment should be provided with a varied diet. When the white blood cell count is low, it is advisable to avoid raw foods that cannot be peeled (e.g. lettuce, strawberries, raw tomatoes).
Sometimes chemotherapy can reduce hunger, or even cause nausea. During the days of chemotherapy, it is not advisable to force your child to eat, as this can be counter-productive.
On the other hand, the corticosteroids (prednisone and dexamethasone) that the child will be administered during some phases of treatment can greatly increase their appetite, even causing anxiety. While they may be allowed to eat more than the meals served in the hospital, they should not be allowed to eat unlimited food, as they will often not tolerate it well and it can cause stomach pain.
Links of interest concerning medical issues relating to acute lymphoblastic leukaemia in children
Childhood Acute Lymphoblastic Leukemia Treatment. National Cancer Institute
Childhood acute lymphoblastic leukaemia (ALL). Cancer Research UK
Childhood acute lymphoblastic leukaemia (ALL). Blood Cancer UK
Acute Lymphoblastic Leukemia (ALL). St Jude Children’s Research Hospital
Links of interest on other topics related to acute lymphoblastic leukaemia in children
CHILDHOOD LEUKAEMIA MATERIAL
- Babies also have leukaemia. Josep Carreras Leukaemia Foundation. (content in Spanish)
- Childhood leukaemia. The little unstoppable ones. Josep Carreras Leukaemia Foundation. (content in Spanish)
- Medulin cut-out set. Josep Carreras Leukaemia Foundation. (content in Spanish)
The Josep Carreras Foundation has a story “The tough baby” aimed at children or siblings suffering from leukaemia. It is especially aimed at children up to the age of 6. If you want to order it, please send us an e-mail to imparables@fcarreras.es.
BONE MARROW TRANSPLANT
- Bone Marrow Transplant Guide. Josep Carreras Leukaemia Foundation.
- What is HLA and how does it work? Josep Carreras Leukaemia Foundation. (content in Spanish)
- Graft-versus-Host Disease Josep Carreras Leukaemia Foundation. (content in Spanish)
- History of Bone Marrow Transplantation. Josep Carreras Leukaemia Foundation. (content in Spanish)
- How is the search for an anonymous donor conducted? Josep Carreras Leukaemia Foundation. (content in Spanish)
- Care guide for transplanted children. TransplantCHild.
- Stem cell transplantation: a colouring book. Leukaemia and Lymphoma Society.
SUPPORT MANUALS
- How to deal with leukaemia and lymphoma in children? Leukemia & Lymphoma Society.
- LIVING BY LEARNING. Action protocol for students with cancer AFANION.
- Support guide for parents of children with cancer ASION.
- A guide for young people and adolescents with cancer ASION.
- Pupils with cancer. A guide for teachers ASION.
- The importance of parental behaviour when a child has cancer ASION.
- My child has cancer. What do I do? FARO.
FOOD
- How to maintain a healthy diet during treatment? Josep Carreras Leukaemia Foundation. (content in Spanish)
- “Bon appetit”. Dietary advice during treatment AFANION.
- ‘Jabel’s magic recipes’. Isabel Rojas Murcia, Carolina Mangas Gallardo.
OTHER
- Information on the long-term and late effects of treatment for leukaemia/lymphoma in children Leukemia & Lymphoma Society.
- My sibling has cancer Josep Carreras Leukaemia Foundation. (content in Spanish)
- School in a hospital Josep Carreras Leukaemia Foundation. (content in Spanish)
- Educating illusions. Guide for psycho-educational intervention in children and adolescents with cancer FARO.
- Cancer in adolescents Josep Carreras Leukaemia Foundation. (content in Spanish)
- ‘Leukaemia and adolescents’ documentary Josep Carreras Leukaemia Foundation.
- ‘Babies also have leukaemia’ documentary Josep Carreras Leukaemia Foundation.
- 7 ways to wear a scarf Josep Carreras Leukaemia Foundation. (content in Spanish)
- ‘Princess Luzie and the chemo knights’ story ASPANAFOA.
- ‘Let’s go to chemotherapy’ story.
- ‘Let’s go to radiotherapy’ story.
- ‘Gasparín Super Quimio’ story Spanish Federation of Parents of Children with Cancer.
- ‘Charlie Brown and leukaemia’ story.
- ‘Toby and the flying machine’ story.
- ‘The fairy of the stars’ story AECC.
- ‘Lina the little swallow’ story Osakidetza.
Useful links: local entities (resources and services)
ANDALUCÍA
ARAGÓN
ASTURIAS
CASTILLA LA MANCHA
CASTILLA LEÓN
CATALUÑA
VALENCIAN COMMUNITY
EXTREMADURA
GALICIA
BALEARIC ISLANDS
CANARY ISLANDS
LA RIOJA
MADRID
- AAA (asociación de adolescentes y Adultos Jóvenes con Cáncer)
- ASION
- FUNDACIÓN CAICO
- FUNDACIÓN ALADINA
- FUNDACIÓN UNOENTRECIENMIL
MURCIA
NAVARRA
BASQUE COUNTRY
Support and assistance
We also invite you to follow us through our main social media (Facebook, Twitter and Instagram) where we often share testimonies of overcoming this disease.
If you live in Spain, you can also contact us by sending an e-mail to imparables@fcarreras.es so that we can help you get in touch with other people who have overcome this disease.
* In accordance with Law 34/2002 on Information Society Services and Electronic Commerce (LSSICE), the Josep Carreras Leukemia Foundation informs that all medical information available on www.fcarreras.org has been reviewed and accredited by Dr. Enric Carreras Pons, Member No. 9438, Barcelona, Doctor in Medicine and Surgery, Specialist in Internal Medicine, Specialist in Hematology and Hemotherapy and Senior Consultant of the Foundation; and by Dr. Rocío Parody Porras, Member No. 35205, Barcelona, Doctor in Medicine and Surgery, Specialist in Hematology and Hemotherapy and attached to the Medical Directorate of the Registry of Bone Marrow Donors (REDMO) of the Foundation).
Become a member of the cure for leukaemia!


