Transparency
Providing clear, detailed and transparent information about our foundation, our actions and the allocation of the contributions entrusted to us is our duty and goes beyond legal requirements
Corporate Governance
The guidelines to be followed by the trustees, the team of professionals and collaborators of the Foundation, with the aim of contributing to its mission, respecting its values and avoiding situations that could harm or damage its reputation, are detailed in:
The Good Governance and Compliance Report is drafted annually to verify its correct implementation.
Regulatory Compliance
Most relevant laws and regulations applicable to the Foundation
- Law 4/2008 of the third book of the Civil Code of Catalonia, relating to legal entities, Law 7/2012, amending the third book of the Civil Code of Catalonia, relating to legal entities and Law 21/2014, on the protectorate of foundations and verification of the activity of public utility associations.
- Law 49/2002, on the tax regime of non-profit organizations and tax incentives for patronage.
- Law 10/2010 on Prevention of Money Laundering and Financing of Terrorism, with a MONEY LAUNDERING PREVENTION MANUAL, describing the established procedures and control measures.
- Organic Law 5/2010, of June 22, introduces for the first time in the Criminal Code an express regulation of the criminal liability of legal entities for crimes committed on their behalf by their representatives, de facto or de jure administrators, workers and/or employees. The reform of the Criminal Code by Organic Law 1/2015, includes the existence of various mitigating and exempting factors for the legal entity, with the so-called crime prevention models or programs being considered a fundamental part of the exemption from criminal liability. CRIMINAL PREVENTION MANUAL.
- Organic Law 3/2018, on Personal Data Protection and guarantee of digital rights, General Data Protection Regulation 2016/679 (GDPR), as well as in Law 34/2002, of July 11, on Information Society Services and Electronic Commerce (LSSI), to ensure compliance with the former, the Foundation carried out a biannual audit which is effectively performed by an external entity.
- Law 15/2022, integral for equal treatment and non-discrimination and all laws on equality, an action protocol has been defined to facilitate assistance for persons affected by a case of harassment.
Most relevant laws and regulations applicable to the REDMO
- The Josep Carreras Foundation maintains and manages the Bone Marrow Donor Registry (REDMO) acknowledged by the Ministry of Health according to the Framework Agreement of June 13, 1994, renewed and currently in force, for the development of the hematopoietic progenitor transplant program of unrelated donors.
- REDMO is integrated in the World Bone Marrow Donor Association Registry REDMO WMDA Qualified in force since February 2019 – The Bone Marrow Donor Registry has obtained the certificate of compliance with the WMDA Standards.
- The REDMO is authorized as a Tissue Establishment as of December 13, 2011 by the Generalitat de Catalunya according to Royal Decree 1277/2003, establishing the general bases on the authorization of health centres, services and establishments and by Royal Decree 9/2014, establishing the quality and safety standards for the donation, procurement, evaluation, processing, preservation, storage and distribution of human cells and tissues and approving the rules of coordination and operation for their use in humans.
- As of March 6, 2023, the Generalitat de Catalunya has granted administrative authorization for the Promotion of Donation.
- Agreements are in place with each of the Autonomous Communities that manage the National Health System and bilateral agreements with donor, collection and transplant centres throughout the territory.
Whistleblower channel
In the event of a breach of regulations by the Foundation and/or its professionals/collaborators, this can be communicated through canaldenuncia@fcarreras.es
The minimum content required consists of the identification of the complainant, a brief description of the facts and the identification of the person or group against whom the complaint is directed.
Once the report has been received by the Regulatory Compliance Officer, the appropriate verifications and checks will be carried out, guaranteeing confidentiality and scrupulously complying with data protection regulations.
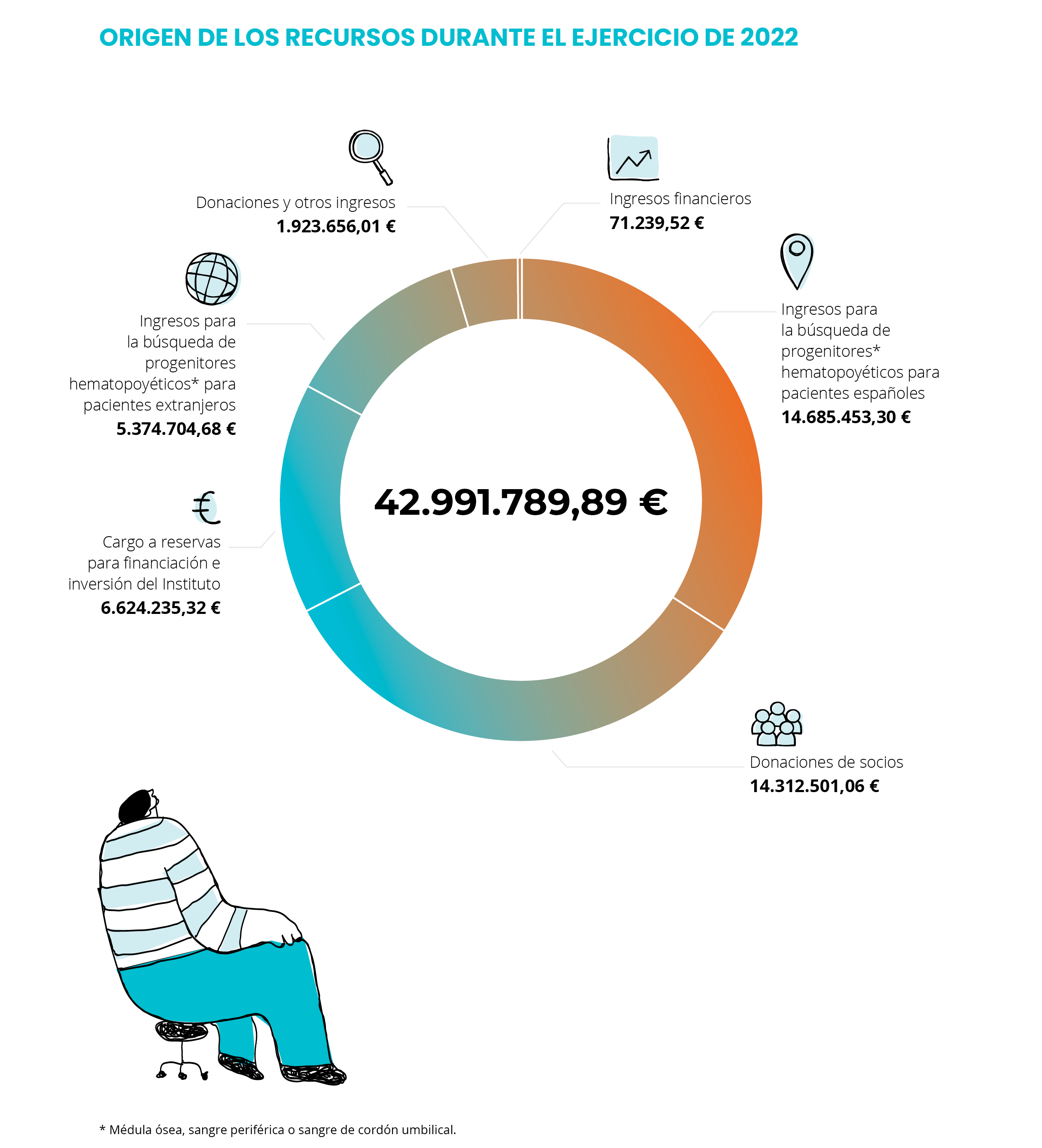
Accounting
Strategic Plan
At the Josep Carreras Foundation, we work following a five-year Strategic Plan to define our objectives and actions applicable to each line of work.
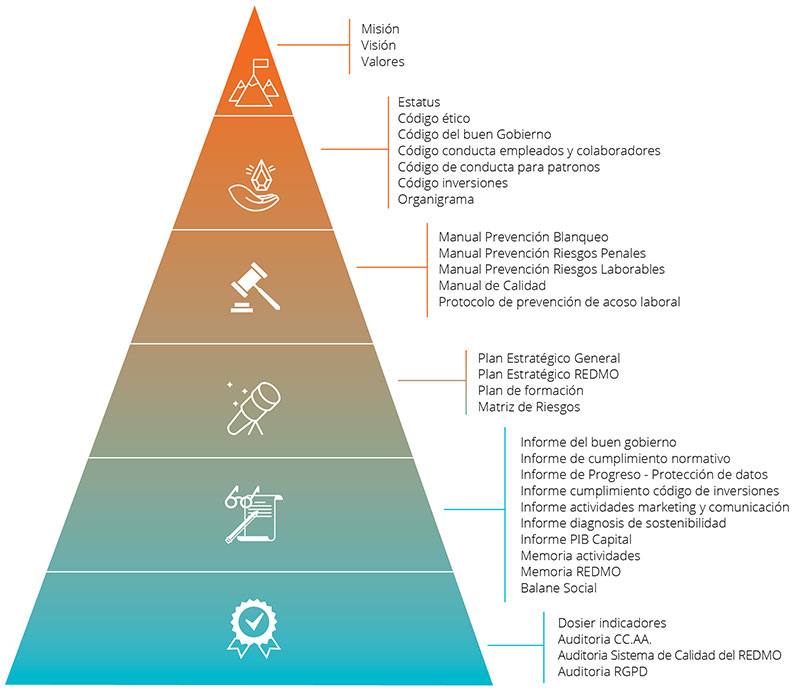
Quality Management System
The Quality Management System implemented at the JOSEP CARRERAS INTERNATIONAL FOUNDATION follows the requirements of ISO 9001 and WMDA standards enabling us to:
- Demonstrate the Foundation’s ability to consistently provide activities that meet the requirements of its stakeholders* and applicable regulatory requirements.
- Increase stakeholder satisfaction always including continuous improvement in its processes.
* by stakeholders we mean: patients, scientific community, health authorities, members, companies, friends, donors, collaborators and society in general.
The Quality Management System abides by the following structure:
Impact measurement system
The Foundation’s mission is carried out by developing its Strategic Plan, and specifically by implementing the three Activity Programs decided by the Board of Trustees.
- Support for scientific research.
- Search for unrelated donors for bone marrow transplants.
- Social services: hospitality apartments, patient care, outreach.
Whether a specific activity is configured as a Foundation Program depends on different criteria:
- Need detected
- Capacity to respond effectively
- Coordination with the public health, research and social services system
- Economic and technical possibilities
This process is carried out by the Board of Trustees with the objective of achieving a positive impact in favour of patients. It involves selecting which activities cannot be undertaken because they are already covered, because they are beyond the reach of resources, because they imply a risk of dispersion of material and human resources and, consequently, because they are not likely to make an appreciable change.
The impact of our Programs is measured in various ways, mainly through quantified and publicized metrics, as well as through accreditations and external evaluations.
Support to scientific research
Scholarships and Grants
The granting, renewal and evaluation of the Scholarships and Grants awarded has been carried out by the National and International Scientific Committees based, fundamentally, on the publications generated.
Impact measurement: The so-called “Impact Factor” and “h Factor” corresponding to each publication, according to standards which are internationally accepted in the scientific community, are accounted for. Currently, this task is carried out by the Technical Secretary of the Foundation.
Josep Carreras Leukaemia Research Institute
Accreditation: The Institute has been positively evaluated (B+ rating) on two occasions (2014 and 2018) by an International Scientific Committee appointed by the CERCA body, of the Generalitat de Catalunya. This evaluation covers both scientific and management aspects: scientific production, talent attraction, professional development, platform and infrastructure management, economic management, impact on the social environment.
Impact measure: The Institute publishes its reports detailing, among other aspects, the fundamental variable that proves the fulfilment of its mission, scientific production. All the information is available in the Transparency section of their website.
The Institute plans to obtain other research quality certificates in the near future that will qualify it for all types of national calls for proposals.
Search for unrelated donors for bone marrow transplantation
Impact measure: The critical parameters of the Registry’s impact are the number of successful unrelated donor searches, as well as the duration of these searches. All these data, their evolution and other quantitative and qualitative indicators are shown in Redmo’s Annual Report and in the Foundation’s Annual Report.
Social services. Hospitality apartments and Patient Care. Dissemination
Hospitality apartments:
–Certificate of good execution
Impact measure: occupancy of the apartments and consultations attended, which are reported in the Annual Report.
Accreditation: Website accredited by the Official College of Physicians of Barcelona and by Health on the Net (HON Code).
Other impact measures
In addition to the impact measures of the programs that develop the foundational mission, the team carries out monthly, quarterly and half-yearly monitoring of all types of indicators, especially those referring to social and economic support, as well as the number of users of our social media channels. The index of the indicators dossier is as follows:
- Summary of results and trends for the period.
- Members: active, registrations and withdrawals during the period, proceeds, classification by recruitment channel, age pyramid, penetration by Autonomous Community, most common amounts, loyalty by channel, return on investment in recruitment by channel, and of; campaigns, recoveries, collections, reactivations; analysis of withdrawals for reasons
- One-off donations: collection, segmentation by type of recruitment channel, age pyramid, segmentation by Autonomous Community.
- Loyalty actions: communication opening rates, analysis by campaign, profitability of actions to increase market share by segment, lead conversion.
- Bequests and inheritances: collection, processing and settlement.
- SMS campaigns: results and indicators of conversion to member
- Charity events: in process, finalized, economic results, repercussion, promotion of new channels.
- Online store: results, sales.
- Corporate alliances: companies sponsoring communication campaigns, variable contributions, partner companies. Recruitment and loyalty. Prospecting actions.
- Communication: issuance of press releases on social media (Facebook, Blog, Twitter, Instagram) and in conventional media, publications achieved, reach, mentions, interactions.
- Social media marketing: web access, origin by country, most visited pages, evolution of followers in media.
- Quality: Updated process map, reviewed processes, improvement projects underway, quality reports, updated risk analysis, progress in ERP implementation.
- Training: contents, participants and evaluations.
- Information technologies: improvement projects, degree of completion.
Translational research. Participation in spin off

Statement by the Josep Carreras International Foundation on its participation in translational research spin-off companies created by the Josep Carreras Leukemia Research Institute.
Become a member of the cure for leukaemia!
































